Catalyst for hydrotreating of boron-containing residual oil and preparation method thereof
A technology of residual oil hydrogenation and catalyst, which is applied in the direction of catalyst activation/preparation, physical/chemical process catalysts, chemical instruments and methods, etc. It can solve the problems of poor stability, unfavorable catalyst active metal dispersion, low nickel removal activity, etc., to achieve Improve hydrogenation performance, facilitate hydrogenation reaction and removal, and weaken the effect of interaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] A preparation method of a boron-containing residue hydrotreating catalyst, the preparation method comprising the steps of:
[0033] Step 1): Prepare the carrier: Prepare the aluminum salt solution, mix the sol by pH swing method, add the organic pore expander solution containing silicon compound during the mixing process, lead it into the gel forming tank, and form it at 60 ° C ~ 90 ° C Glue, after gelation, aging, drying, molding, and then roasting at 550°C to 1100°C to obtain an alumina carrier; the pH swing range is 4 to 10, and the pH of the final reaction system is 8 to 10;
[0034] Step 2): impregnating the alumina support with an equal volume of Mo or W and Co or Ni impregnation solution, then drying at 90-150°C for 2-8 hours, and then calcining at 600-800°C for 1-5 hours , to obtain a hydrodemetallization catalyst;
[0035] The hydrodemetallization catalyst contains MoO 3 or WO 3 and NiO or CoO, the MoO 3 or WO 3 The total mass of NiO or CoO accounts for 5....
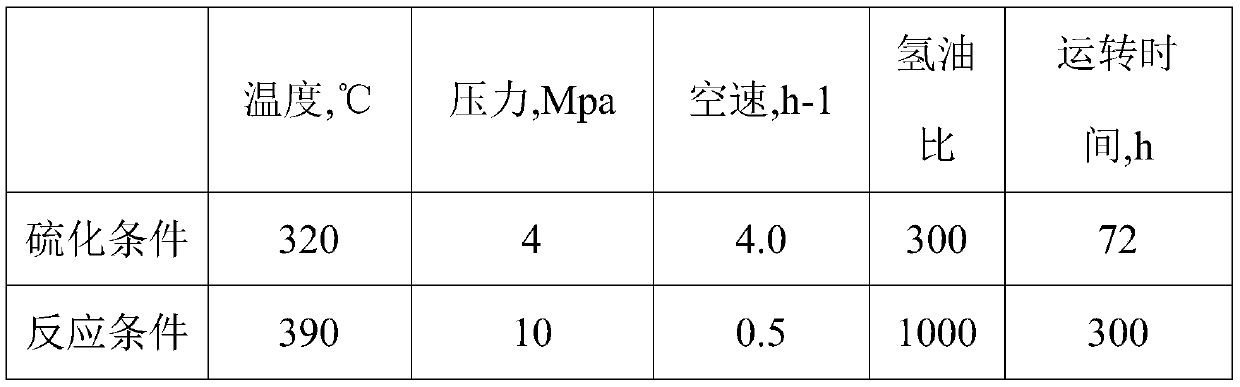
Embodiment 1
[0047] (1) Preparation of carrier: prepare 0.98M aluminum chloride solution, and under stirring conditions, add the above aluminum chloride solution and ammonia water into the gelling tank in parallel using the pH swing method, and the flow rate of aluminum chloride is 2.0 ml / min, the temperature in the glue tank is controlled at 70°C, the pH swing range is 4-10, and the pH of the final reaction system is 8-10. At the same time, 2.0 ml / min of a polystyrene ball pore-enlarging agent solution containing a certain amount of boric acid was added, and the total amount of the pore-enlarging agent added was 13% of the mass of the calcined alumina. After gelling, aging for 0.5h, drying at 50°C for 60h under vacuum condition, extruding and sintering at 800°C for 6h, the B-loaded 2 o 3 (accounting for 1.5w% of final catalyst content) alumina carrier B 2 o 3 / γ~Al 2 o 3 .
[0048] (2) Impregnation of active metals: according to the MoO in the final catalyst 3 The content of NiO is...
Embodiment 2
[0050] (1) Preparation of the carrier: prepare 1.0M aluminum chloride solution, under stirring conditions, add the above aluminum chloride solution and ammonia water into the gelling tank in parallel using the pH swing method, and the flow rate of aluminum chloride is 2.0 ml / min, the temperature in the glue tank is controlled at 70°C, the pH swing range is 4-10, and the pH of the final reaction system is 8-10. At the same time, 2.0 ml / min of a polystyrene ball pore-enlarging agent solution containing a certain amount of boric acid was added, and the total amount of the pore-enlarging agent added was 15% of the mass of the calcined alumina. After gelling, aging for 0.5h, drying at 50°C for 60h under vacuum, extruding and sintering at 800°C for 6h, the alumina carrier B loaded with B2O3 (accounting for 2.0w% of the final catalyst content) was obtained 2 o 3 / γ~Al 2 o 3 .
[0051] (2) Impregnation active metal: according to MoO in the final catalyst content 9.2wt%, the conten...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com