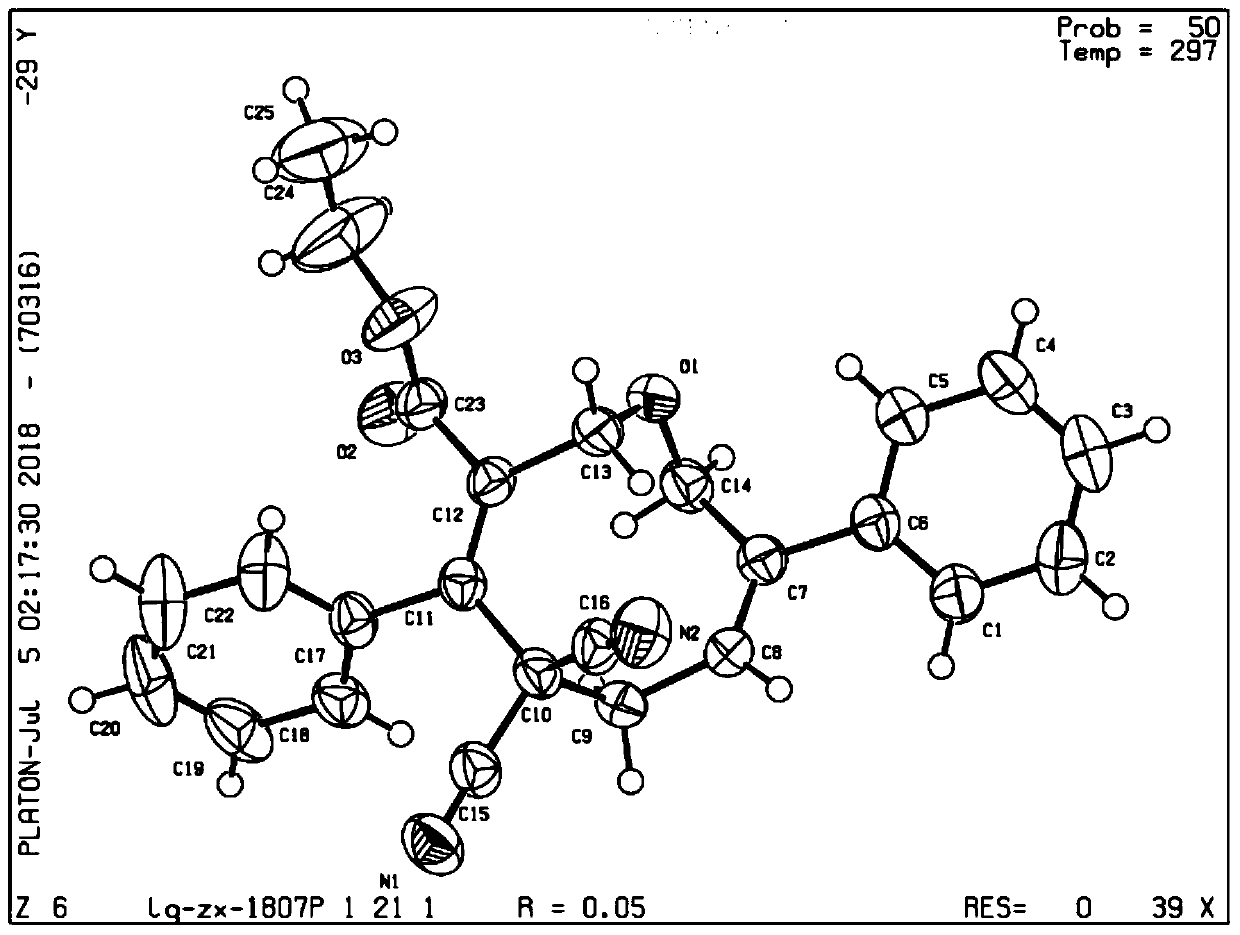
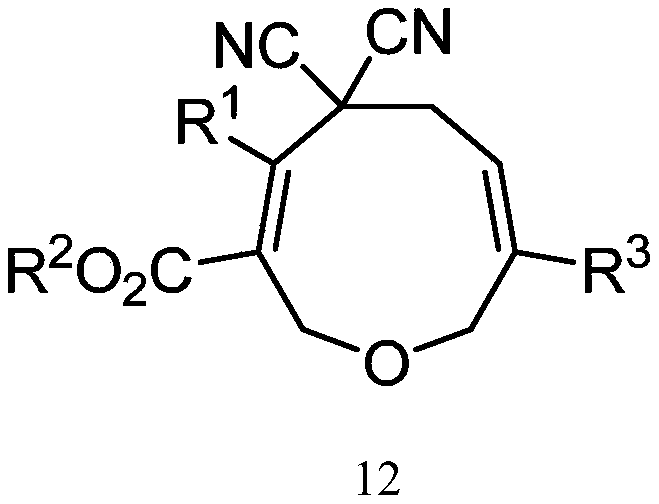
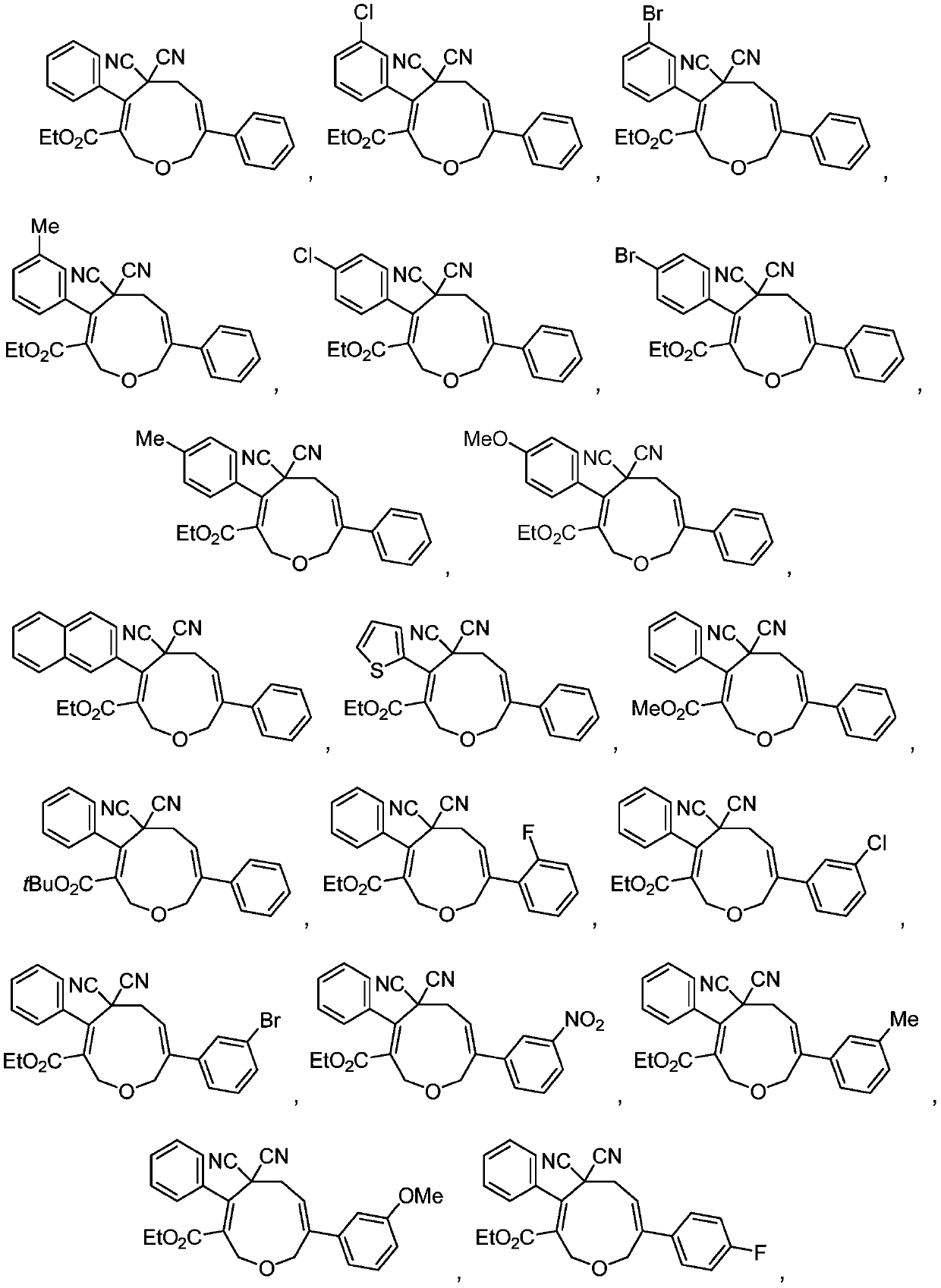
Oxacyclononadiene derivative, and pharmaceutical composition, preparation method and application thereof
An oxygen heterocycle, nonadiene technology, applied in the directions of drug combination, pharmaceutical formula, organic chemical method, etc., can solve the problems of limited industrial production, complex production process, low product yield, etc., achieve good anti-tumor effect, broad The effect of market application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Compound: (3E,7Z)-5,5-Dicyano-4,8-diphenyl-2,5,6,9-tetrahydrooxacyclononadiene-3-ethyl acetate
[0069]
[0070] Preparation:
[0071] S1, preparation of dicyanodiene 5:
[0072] Step 1: Put benzaldehyde 1 (1.0eq) into a 250mL round-bottomed flask, add distilled water (100mL) as a solvent, put in a clean stirring bar, then add malononitrile (1.2eq), and react at room temperature for 12h . After the reaction was detected by TLC, a large amount of solids were precipitated, filtered, the filter cake was washed with petroleum ether, and the white solid product benzylidene malononitrile 3 was dried;
[0073] Step 2: Weigh benzylidene malononitrile 3 (1.0eq), catalyst PPh 3 (0.2eq) was added to a 250mL round-bottomed flask with a stirring magnet, and a reaction device with a constant pressure dropping funnel was assembled. Under the protection of argon, accurately measure 25mL of toluene into the round-bottomed flask with a syringe, and then accurately measure Ethyl pr...
Embodiment 2
[0088] Compound: (3E,7Z)-4-(3-chlorophenyl)-5,5-dicyano-8-phenyl-2,5,6,9-tetrahydrooxacyclononadiene-3- ethyl acetate
[0089]
[0090] Preparation:
[0091] The difference with the preparation method of embodiment 1 is:
[0092] (1) In step S3, the reaction catalyst is palladium tetrakistrifurylphosphine; the reaction temperature is 15°C; the molar ratio of palladium tetrafurylphosphine, dicyanodiene 5 and the alkenyl cyclic carbonate 11 satisfies: 0.04:0.8:1.2; The reaction time is 20h; The reaction solvent is dichloromethane;
[0093] (2) Compound 1 is 3-chlorobenzaldehyde, compound 4 is ethyl propiolate, and compound 6 is acetophenone; the product is a light yellow solid with a yield of 69%;
[0094] 1 H NMR (600MHz, CDCl 3 )δ (ppm): 7.54 (d, J = 7.8Hz, 2H), 7.41–7.36 (m, 4H), 7.33 (t, J = 8.4Hz, 1H), 7.20 (s, 1H), 7.10 (d, J=7.2Hz, 1H), 6.24(t, J=9.0Hz, 1H), 4.61(s, 2H), 4.56(d, J=4.8Hz, 2H), 3.91(q, J=6.0Hz, 2H) ,3.62(d,J=11.4Hz,2H),0.87(t,J=7.2Hz,3H).
[0095...
Embodiment 3
[0097] Compound: (3E,7Z)-4-(3-bromophenyl)-5,5-dicyano-8-phenyl-2,5,6,9-tetrahydrooxacyclononadiene-3- ethyl acetate
[0098]
[0099] Preparation:
[0100] The difference with the preparation method of embodiment 1 is:
[0101] (1) In step S3, the reaction catalyst is tetrakistributylphosphine palladium; the reaction temperature is 22°C; the molar ratio of tetrakistributylphosphine palladium, dicyanodiene 5 and the alkenyl cyclic carbonate 11 satisfies : 0.06:1.5:2.2; the reaction time is 30h;
[0102](2) Compound 1 is 3-bromobenzaldehyde, compound 4 is ethyl propiolate, and compound 6 is acetophenone;
[0103] The product is a yellow semi-solid with a yield of 53%;
[0104] 1 H NMR (600MHz, CDCl 3 )δ(ppm):7.59–7.51(m,3H),7.44–7.36(m,3H),7.36–7.32(m,1H),7.28(d,J=8.4Hz,1H),7.15(d,J =7.8Hz, 1H), 6.23(t, J=9.0Hz, 1H), 4.60(s, 2H), 4.56(d, J=4.2Hz, 2H), 3.91(q, J=7.2Hz, 2H), 3.62(dd,J=9.0,5.4Hz,2H),0.87(t,J=7.2Hz,3H).
[0105] 13 C NMR (150MHz, CDCl 3 )δ (ppm): 167....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com