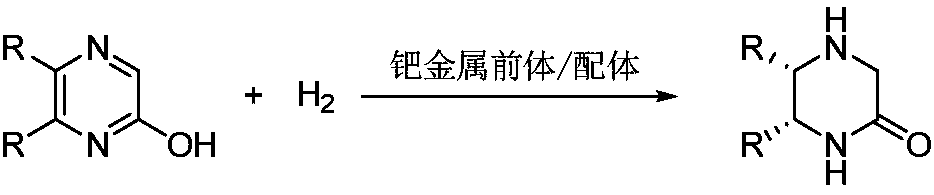
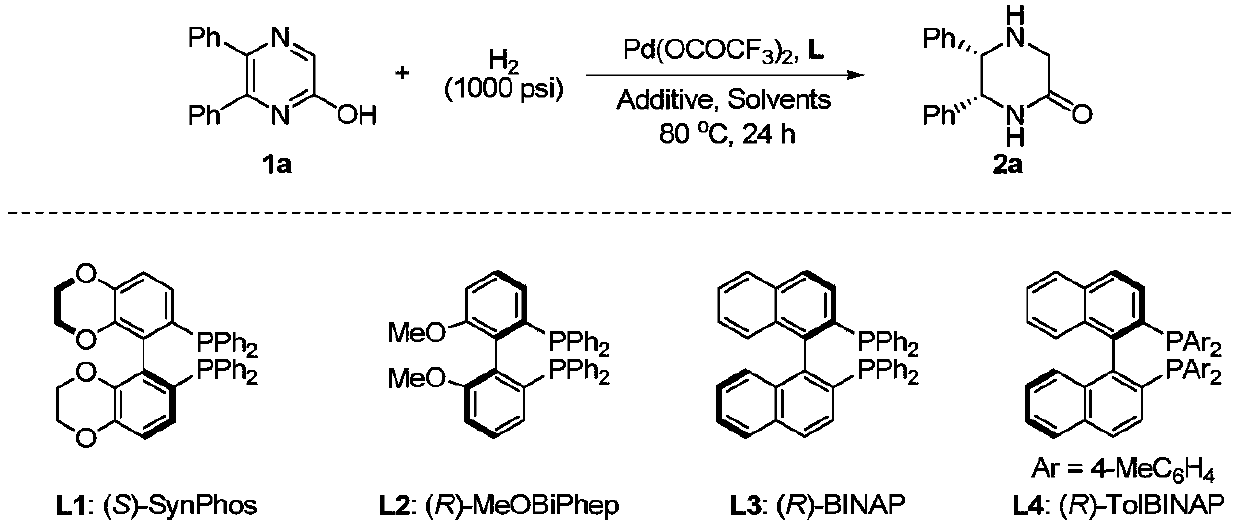
Method of asymmetric hydrogenation synthesis of chiral lactam by catalyzing 2-hydroxypyrazine compound with palladium
A hydroxypyrazine and asymmetric technology, which is applied in the field of chemical synthesis of chiral cyclic amines, can solve the problems of cumbersome steps and limited substrate range, and achieve good enantioselectivity, good air stability and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-12
[0030] Optimization of Hydrogenation Conditions of 5,6-Disubstituted-2-Hydroxypyrazine
[0031] Drop into palladium trifluoroacetate (1mol%-5mol% of substrate consumption) and chiral bisphosphine ligand (1.1mol%-5.5mol% of substrate consumption) in reaction flask, add organic solvent acetone (1.0mol%) after nitrogen displacement -4.0mL), stirred at room temperature for 30 minutes, and removed the organic solvent under reduced pressure; then, in the glove box, this catalyst was transferred to pre-placed substrate 1a (0.2mmol) and additive ( 10mol%-100mol% of the amount of substrate) in a reaction flask, moved to a reaction kettle, introduced hydrogen (400psi-1200psi), and reacted at 40-100 degrees Celsius for 24 hours; released hydrogen, and directly separated by column chromatography after removing the solvent To obtain the target product, change the types of organic solvents, additives, and chiral bisphosphine ligands in the reaction process, and obtain 12 different examples....
Embodiment 13-24
[0038] Synthesis of Chiral Lactams by Palladium-Catalyzed Asymmetric Hydrogenation of 5,6-Disubstituted-2-Hydroxypyrazines
[0039]Put palladium trifluoroacetate (3.0 mol% of the substrate amount) and (R)-TolBINAP (3.3 mol% of the substrate amount) into the reaction flask, add organic solvent acetone (1.0mL) after nitrogen replacement, and stir at room temperature for 30 minutes , and the solvent was removed under reduced pressure. Then, in the glove box, this solution was transferred to an ampoule containing substrate 1 (0.3 mmol) and p-toluenesulfonic acid monohydrate (100 mol%) with dichloromethane and benzene (1.5 mL / 1.5 mL). , moved to the reaction kettle, feed hydrogen (1000psi), and reacted for 24 hours at 80 degrees Celsius; release hydrogen, remove the solvent and separate directly to obtain pure products by column chromatography, change the type of substrate in the reaction process, and obtain 12 Different embodiments, the types of changes are shown in Table 2. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com