Acidic dye for polyamide fiber dyeing and preparation method thereof
A technology of acid dyes and nylon fibers, applied in azo dyes, organic dyes, monoazo dyes, etc., can solve problems such as dark color of metal complex dyes, human health hazards, non-bright fibers, etc., to solve the problem of heavy metals on the environment The effect of pollution, bright shade, excellent wet fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] C 17 H 24 N 4 O 3 S, molecular weight: 364.46] preparation method, followed by the following steps:
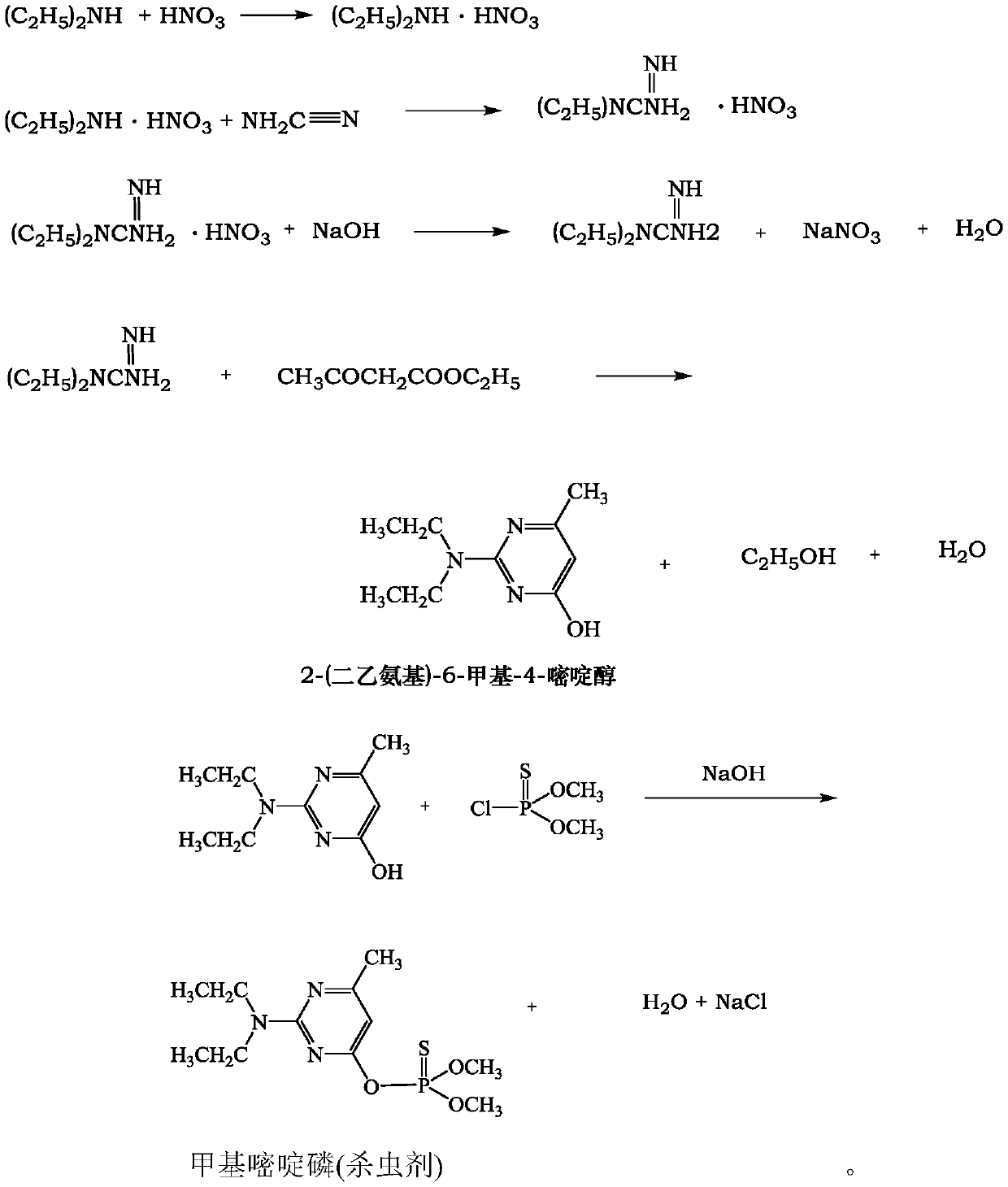
[0043] 1) Synthesis of 2-(diethylamino)-6-methyl-4-pyrimidinol
[0044] In 250mL of water, slowly add nitric acid (9.45g, 0.15mol) dropwise (dropping time is about 10 minutes), and then dropwise add diethylamine (10.22g, 0.14mol) (dropping time about 30 minutes), stirring 1.5 After h, cyanamide (12.60g, 0.3mol) was added dropwise (dropping time is about 30 minutes), and then the temperature was raised to 75°C and reacted for 5 hours. Immediately after the reaction, the temperature of the reactant was cooled to below 5°C with an ice water bath, cooled to precipitate, filtered, and the filter cake was dried at 80°C to a constant weight to obtain a white solid (diethylguanidine nitrate). Dissolve the white solid in a mixed solvent consisting of 100ml of toluene and 30ml of ethanol, add 6g of NaOH (0.16mol) to control the temperature at 30°C, add 20.80g (0.16mol) of ethyl aceto...
Embodiment 1
[0053] 1) Diazotization:
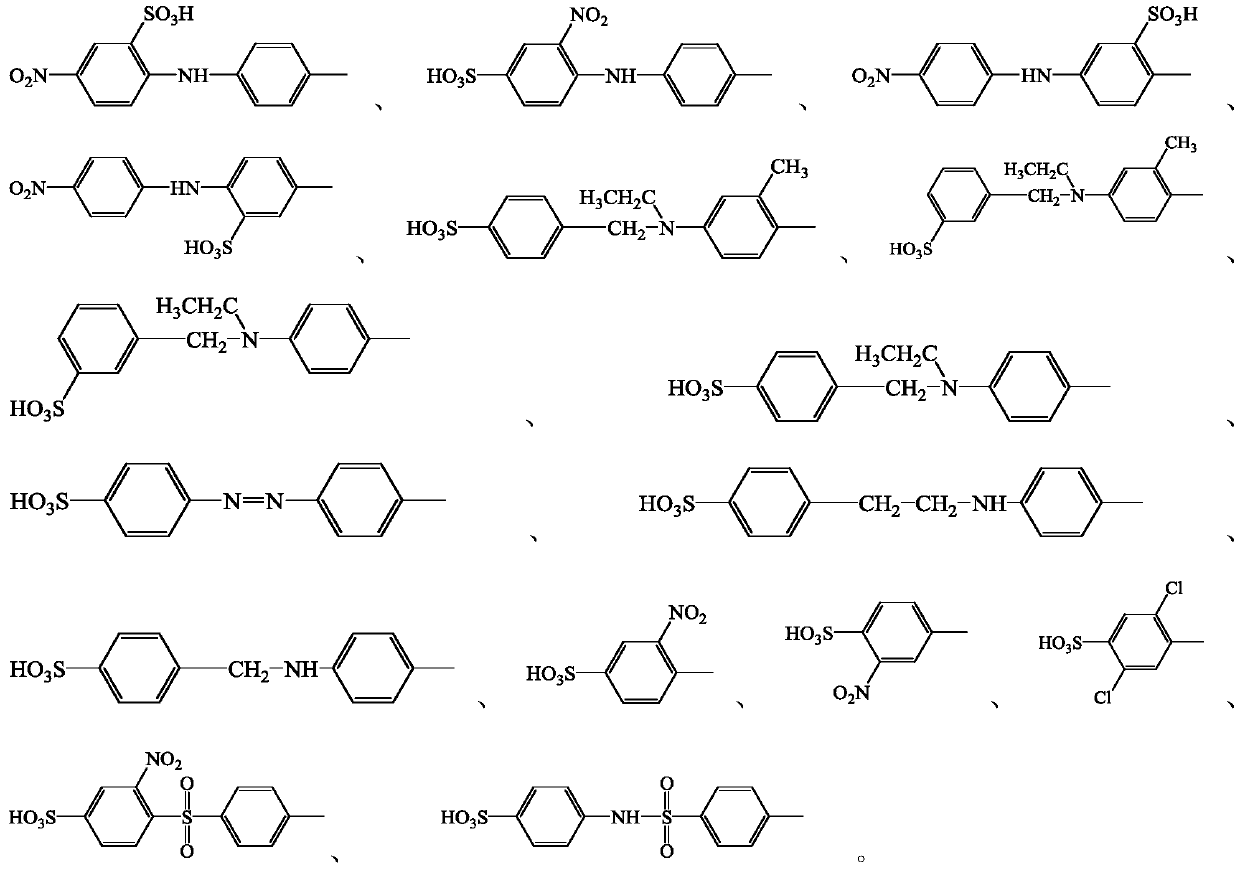
[0054] 2-((4-aminophenyl)amino)-5-nitrobenzenesulfonic acid [2-((4-aminophenyl)amino)-5-nitrobenzenesulfonic acid; molecular formula: C 12 H 11 N 3 O 5 S; molecular weight: 309.30] 30.93g (0.1mol) dissolved in 400ml of water, adjust pH=7.5 with 10% (mass%) sodium hydroxide aqueous solution, add 7g (0.104mol) of sodium nitrite and stir until the dissolution is complete, ice bath Cool down to 0°C, then add to a solution consisting of 200g crushed ice and 26g 30% (0.21mol) hydrochloric acid. The addition time is 30 minutes. During the addition, the temperature is controlled to 0~5℃. After the addition is completed, the temperature is 0~5℃. Keep the temperature and stir for 60 minutes, the diazotization is completed, and the obtained diazonium salt solution is ready for use.
[0055] 2), coupling
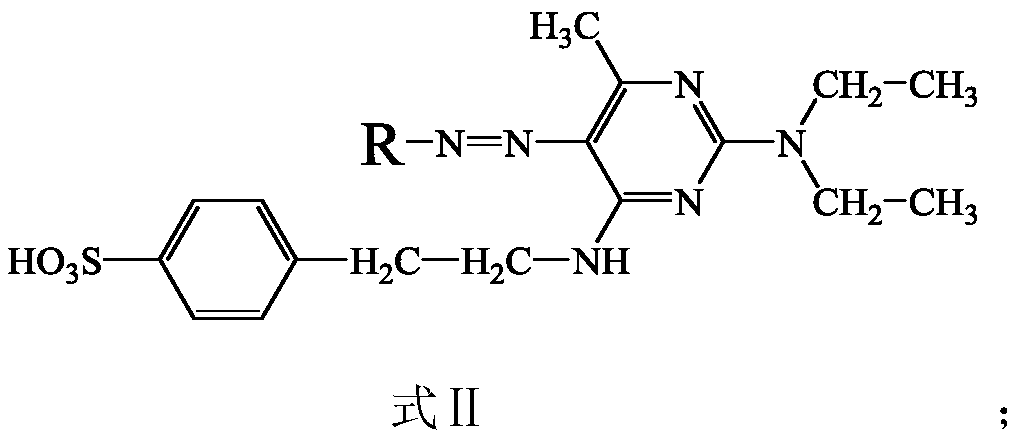
[0056] 4-(2-((2-(Diethylamino)-6-methylpyrimidin-4-yl)amino)ethyl)benzenesulfonic acid [4-(2-((2-(diethylamino)-6- methylpyrimidin-4-yl)amino)ethyl)benzenesulfonic...
Embodiment 2~4
[0061] The 2-((4-aminophenyl)amino)-5-nitrobenzenesulfonic acid in the step 1) of Example 1 was separately used with 3-nitro-4-(4-aminophenylamino)benzenesulfonic acid [English name: 4-((4-aminophenyl)amino)-3-nitrobenzenesulfonicacid, molecular formula: C 12 H 11 N 3 O 5 S, molecular weight: 309.30], 30.93g (0.1mol), 2-amino-5-((4-nitrophenyl)amino)benzenesulfonic acid [English name: 2-amino-5-((4-nitrophenyl) amino)benzenesulfonic acid, molecular formula: C 12 H 11 N 3 O 5 S Molecular weight: 309.30], 30.93g (0.1mol), 5-nitro-2((nitrophenyl)amino)benzenesulfonic acid [English name: 5-amino-2-((4-nitrophenyl)amino)benzenesulfonic acid , Molecular formula: C 12 H 11 N 3 O 5 S molecular weight: 309.30] 30.93g (0.1 mol) instead, the molar amount remains unchanged; the rest is the same as in Example 1; thus, the following dyes with chemical structures II-2, II-3, and II-4 are obtained:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com