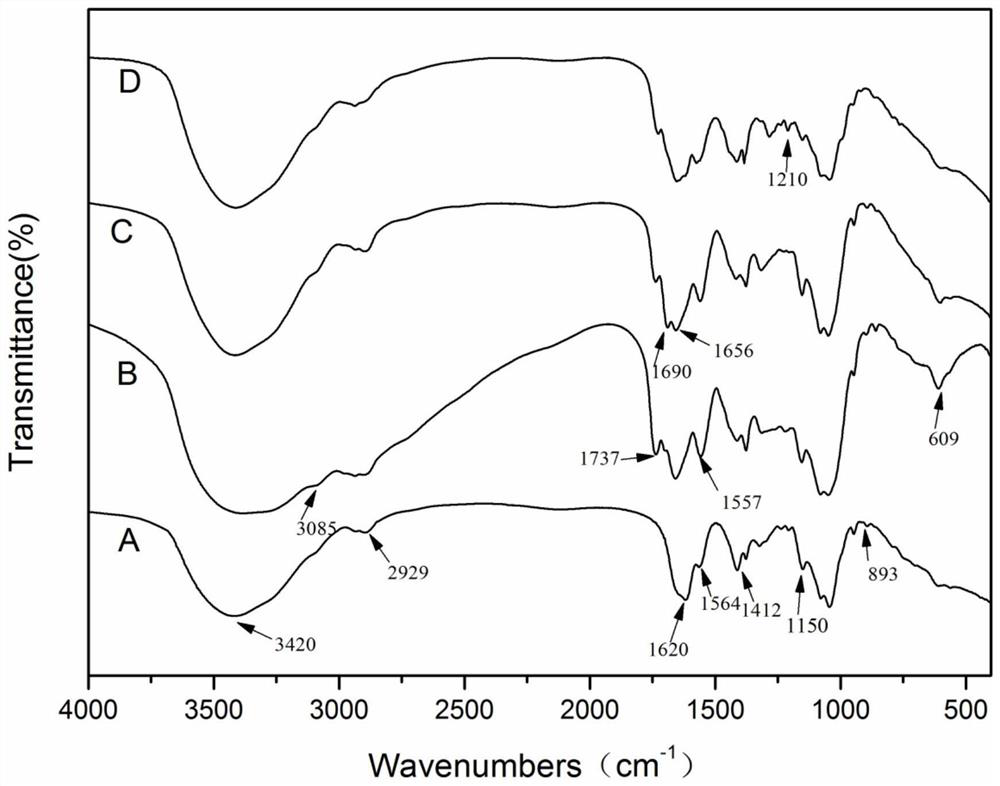
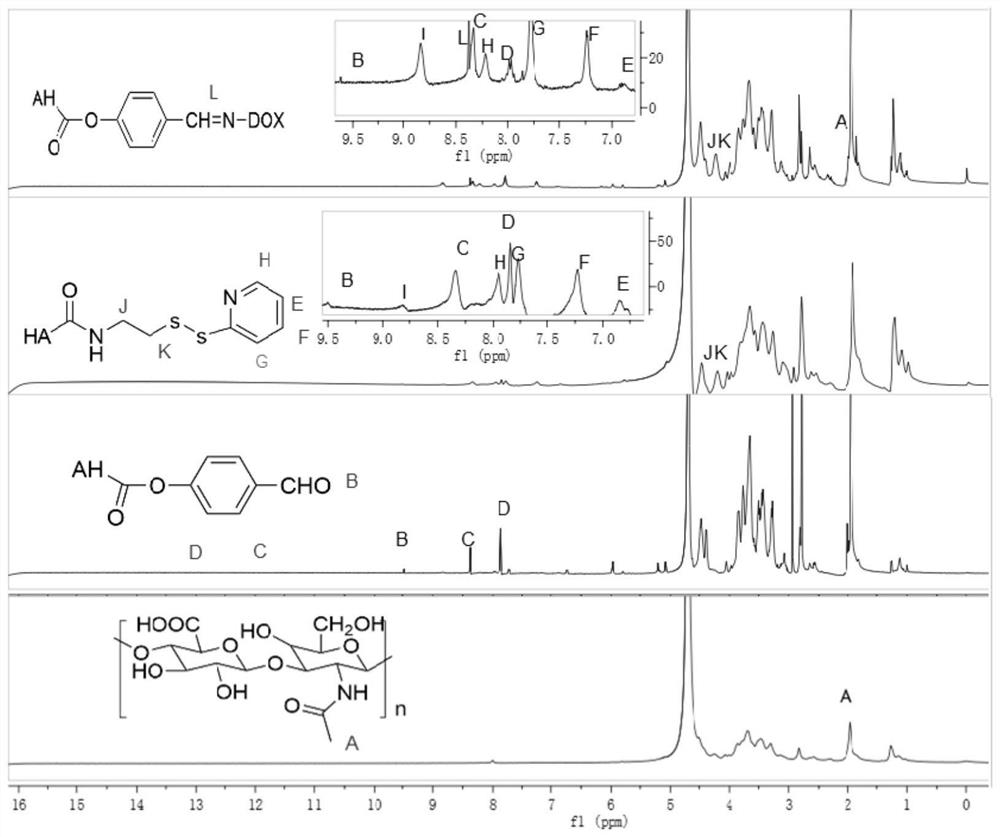
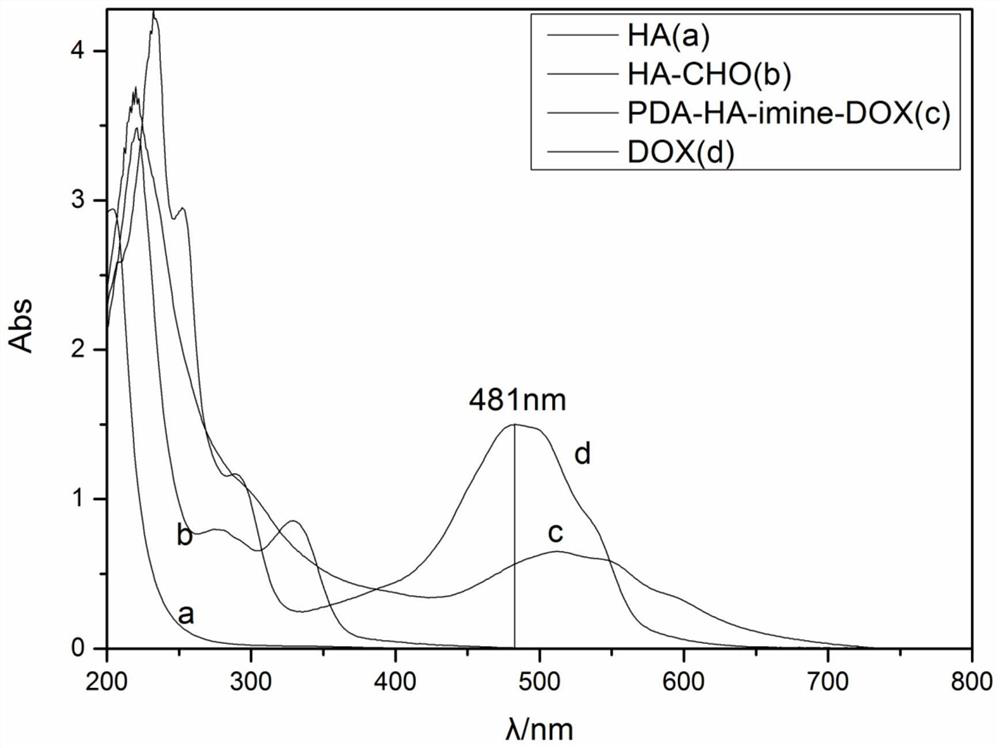
Preparation method and application of doxorubicin-methotrexate combined drug delivery nano-delivery system
A technology of methotrexate and delivery system, which is applied in the direction of medical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems of chemical resistance mutation, tumor recurrence, etc., and achieve the goal of reducing toxicity. Side effects, broad application prospects, and the effect of improving passive targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) Dissolve 882mg (4mmol) of 2,2-dithiobipyridine in methanol (4.16mL) containing 160μL of glacial acetic acid, inject it into an eggplant-shaped bottle filled with nitrogen, and then add 228mg (2mmol) of mercaptoethylamine hydrochloride The salt was dissolved in 1.75mL of methanol and slowly added dropwise to the above solution, and stirred at 35°C for 48h. Concentrate under reduced pressure after the completion of the reaction to obtain 3-5 mL of yellow oil, add 20 mL of cold ether to obtain a precipitate, then dissolve in 5 mL of methanol, continue to precipitate with 20 mL of cold ether until no oily matter, and dry in vacuo to obtain a white powder, namely is PDA·HCl.
[0048] (2) Dissolve 100mg (0.263mmol) of hyaluronic acid (HA, MW=5300Da) in 5mL of formamide, then add 100mg (0.526mmol) of EDC·HCl to the above solution while stirring, and add 60.53mg after 1h (0.526mmol) NHS, activated at room temperature for 1h, another 318.59mg (2.63mmol) p-hydroxybenzaldehyd...
Embodiment 2
[0059](1) Dissolve 882mg (4mmol) of 2,2-dithiobipyridine in methanol (4.16mL) containing 160μL of glacial acetic acid, inject it into an eggplant-shaped bottle filled with nitrogen, and then add 228mg (2mmol) of mercaptoethylamine hydrochloride The salt was dissolved in 1.75mL of methanol and slowly added dropwise to the above solution, and stirred at 35°C for 48h. Concentrate under reduced pressure after the completion of the reaction to obtain 3-5 mL of yellow oil, add 20 mL of cold ether to obtain a precipitate, then dissolve in 5 mL of methanol, continue to precipitate with 20 mL of cold ether until no oily matter, and dry in vacuo to obtain a white powder, namely is PDA·HCl.
[0060] (2) Dissolve 100mg (0.263mmol) of hyaluronic acid (HA, MW=5300Da) in 5mL of formamide, then add 100mg (0.526mmol) of EDC·HCl to the above solution while stirring, and add 60.53mg after 1h (0.526mmol) NHS, activated at room temperature for 1h, another 318.59mg (2.63mmol) p-hydroxybenzaldehyde...
Embodiment 3
[0066] (1) Dissolve 882mg (4mmol) of 2,2-dithiobipyridine in methanol (4.16mL) containing 160μL of glacial acetic acid, inject it into an eggplant-shaped bottle filled with nitrogen, and then add 228mg (2mmol) of mercaptoethylamine hydrochloride The salt was dissolved in 1.75mL of methanol and slowly added dropwise to the above solution, and stirred at 35°C for 48h. Concentrate under reduced pressure after the completion of the reaction to obtain 3-5 mL of yellow oil, add 20 mL of cold ether to obtain a precipitate, then dissolve in 5 mL of methanol, continue to precipitate with 20 mL of cold ether until no oily matter, and dry in vacuo to obtain a white powder, namely is PDA·HCl.
[0067] (2) Dissolve 100mg (0.263mmol) of hyaluronic acid (HA, MW=9800Da) in 5mL of formamide, then add 100mg (0.526mmol) of EDC·HCl to the above solution while stirring, and add 60.53mg after 1h (0.526mmol) NHS, activated at room temperature for 1h, another 318.59mg (2.63mmol) p-hydroxybenzaldehyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com