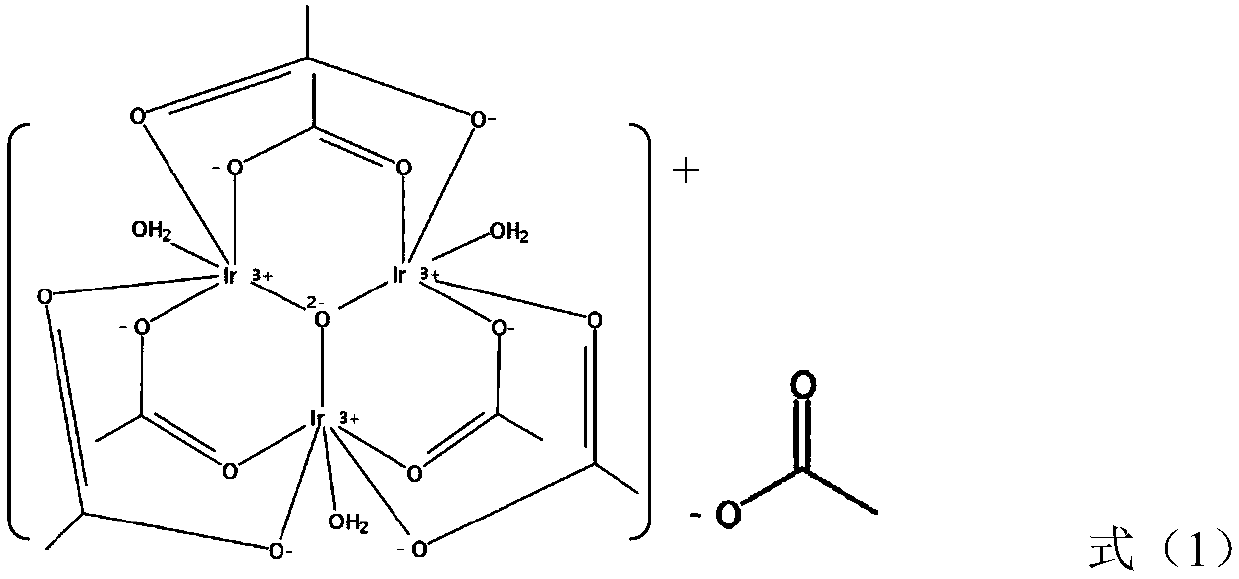
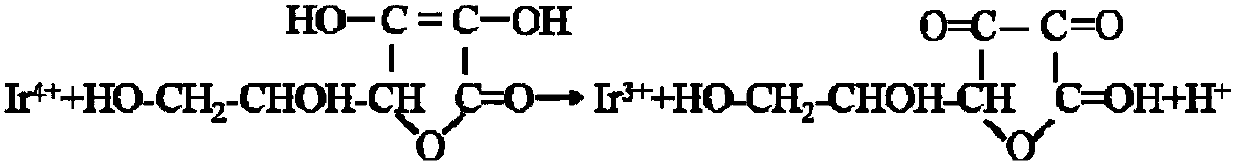Preparation method of iridium acetate
A kind of technology of iridium acetate and iridium hydroxide, applied in the field of catalyst preparation, can solve the problems such as not taking into account the problem of iridium valence, the increase of impurity content, the reduction of iridium acetate yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-3
[0060] Wherein, the content of iridium in the product iridium (III) acetate was measured by inductively coupled plasma mass spectrometry (ICP) (model Agilent 7500cx).
[0061] The product yield was calculated by formula (2).
[0062] Product yield = (m t ×w / m i )×100% Formula (2)
[0063] Among them, m t - product quality; The content of the iridium in the w-iridium acetate (III) powder; m i - the mass of iridium in the aqueous solution containing iridium(IV).
Embodiment 1
[0066] Weigh 5g of chloroiridic acid and put it in a three-necked flask, add 50ml of deionized water to dilute. Under the condition of stirring, pass high-purity nitrogen gas (gas flow rate: 100ml / min) into the solution to purge for 30 minutes to obtain an aqueous solution containing iridium (IV).
[0067] Afterwards, 2.2 g of ascorbic acid was added to the aqueous solution containing iridium (IV), and the solution was heated to 98 ° C under stirring conditions, and refluxed at this temperature for 3 h to complete the reduction treatment and obtain an aqueous solution of iridium (III).
[0068] Then, the aqueous solution of iridium (Ⅲ) is cooled to 60 ℃, and in the aqueous solution of iridium (Ⅲ), sodium hydroxide solution (mass concentration is 30%) is added dropwise, and the pH of the aqueous solution of iridium (Ⅲ) is monitored during the dropping process value. When the pH value of the aqueous solution of iridium (Ⅲ) increased to 11, stop the dripping of sodium hydroxide ...
Embodiment 2
[0073] Weigh 5g of chloroiridic acid and put it in a three-necked flask, add 50ml of deionized water to dilute. Under the condition of stirring, high-purity nitrogen gas (gas flow rate: 200ml / min) was passed into the solution for purging for 30 minutes to obtain an aqueous solution containing iridium (IV).
[0074] Afterwards, 0.9 g of hydrazine hydrate was added to the aqueous solution containing iridium (IV), and the solution was continued to be heated to 98° C. with stirring, and refluxed at this temperature for 3 h to complete the reduction treatment and obtain an aqueous solution of iridium (III).
[0075] Then, the aqueous solution of iridium (Ⅲ) is cooled to 80 ℃, and in the aqueous solution of iridium (Ⅲ), sodium hydroxide solution (mass concentration is 30%) is added dropwise, and the pH of the aqueous solution of iridium (Ⅲ) is monitored during the dropping process value. When the pH value of the iridium (III) aqueous solution increased to 11, the dropwise addition ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com