Vero cell low-serum culture medium and application thereof
A low serum and culture medium technology, applied in cell culture active agent, animal cells, culture process, etc., can solve the problems affecting the stability and quality of vaccine production process, the effect of downstream purification of cell growth, and the difference between serum batches, etc. Eliminate instability, reduce the complexity of purification process, and clarify the effect of ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
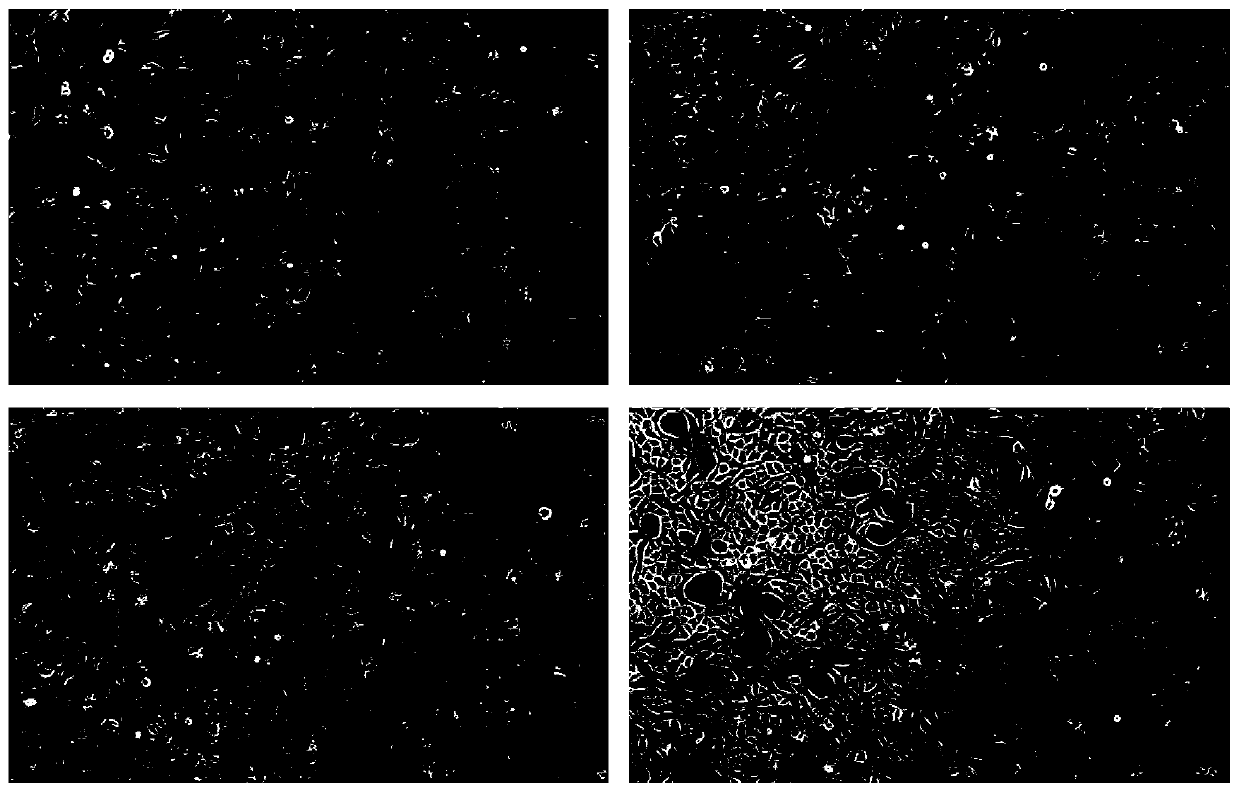
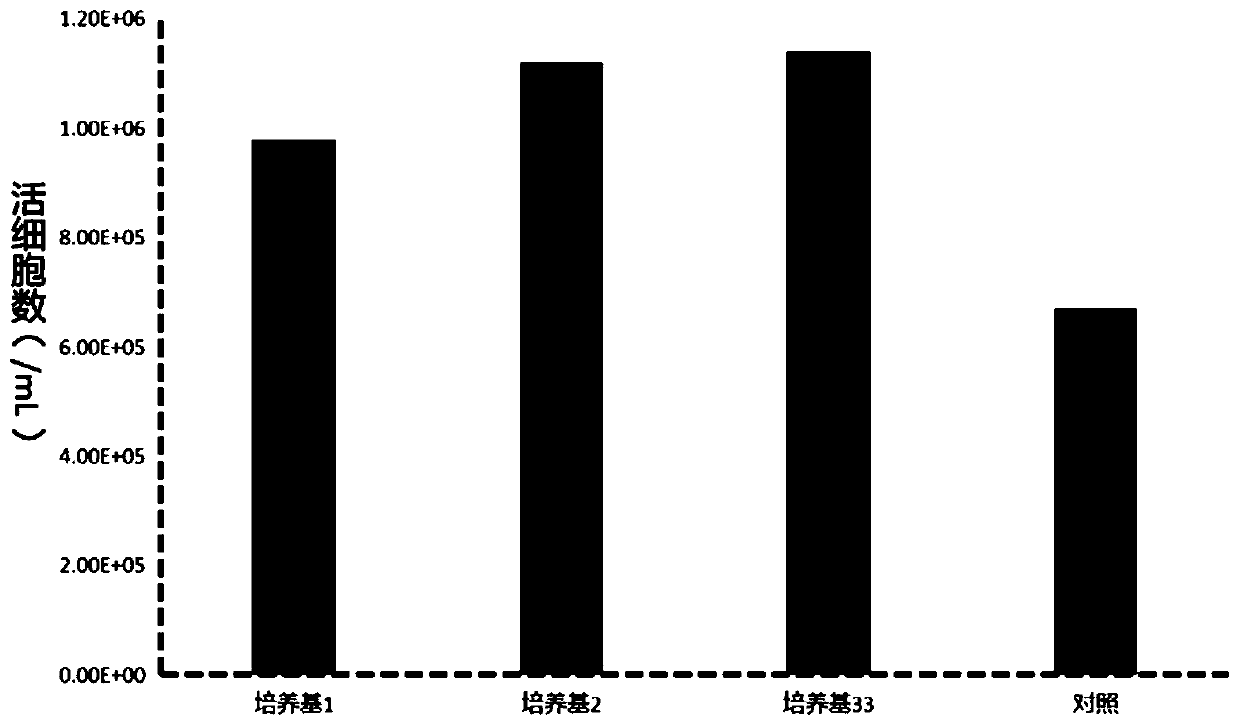
[0034] Example 1 Vero cell low serum medium (1)
[0035] Includes basal medium and supplementary components:
[0036] Added components: EDTA disodium 1mg / L, dihydrate ethanolamine hydrochloride 5mg / L, EDTA iron sodium 2mg / L, rhEGF 0.005mg / L, inositol 50mg / L, vitamin B60 .5mg / L, vitamin B70.01mg / L;
[0037] Basal medium: DMEM / F12.
Embodiment 2
[0038] Embodiment 2 Vero cell low serum culture medium (two)
[0039] Includes basal medium and supplementary components:
[0040] Added components: EDTA disodium 10mg / L, dihydrate ethanolamine hydrochloride 30mg / L, EDTA iron sodium 25mg / L, rhEGF 0.02mg / L, inositol 80mg / L, vitamin B65mg / L, vitamin B70.3mg / L;
[0041] Basal medium: DMEM / F12.
Embodiment 3
[0042] Example 3 Vero Cell Low Serum Medium (3)
[0043] Includes basal medium and supplementary components:
[0044] Added components: disodium EDTA 1mg / L, ethanolamine dihydrate hydrochloride 30mg / L, iron sodium EDTA 2mg / L, rhEGF 0.02mg / L, inositol 50mg / L, vitamin B65mg / L, vitamin B70.01mg / L;
[0045] Basal medium: DMEM / F12.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com