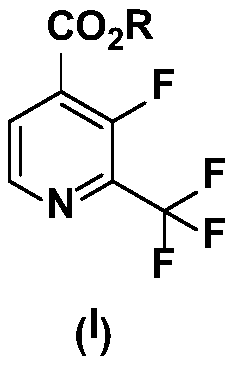
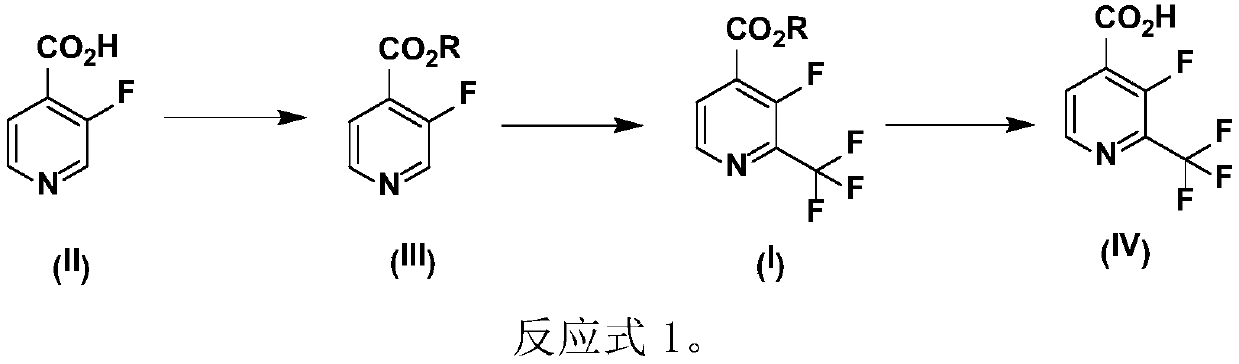
Preparation method of 2-trifluoromethyl-3-fluoro-4-picolinic acid and derivatives thereof
A technology of sodium trifluoromethyl sulfinate and picolinic acid, which is applied in the field of medicine and chemical industry, can solve the problems of high price of Meiben reagent, insufficient atom economy, positional selectivity, etc., and achieve high selectivity, high conversion rate, and raw material Easy to get effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1. Preparation of methyl 2-trifluoromethyl-3-fluoro-4-pyridinecarboxylate (VI), as shown in Reaction Formula 4.
[0040]
[0041] In a 5L three-necked reaction flask, add 1.25L of dichloromethane, 500mL of water and the compound 3-fluoro-4-pyridinecarboxylic acid methyl ester (250g, 1.0eq) represented by formula (V) and stir evenly, then add 250mL of tert-butanol peroxide and 360 g of sodium trifluoromethylsulfinate (5.0 eq). The mixture was stirred at room temperature for 12 hours and monitored for completion.
[0042] The dichloromethane solvent was carefully removed at room temperature, and the residue was carefully added to crushed ice, stirred evenly, and extracted twice with diethyl ether. After the combined organic phases were washed three times with ice water, the organic phases were dried and concentrated at low temperature, and the obtained product (VI) was directly put into the next reaction.
Embodiment 2
[0044] 1. Preparation of methyl 3-trifluoromethyl-4-pyridinecarboxylate (V), as shown in Reaction Formula 3.
[0045]
[0046] In a 100L reactor, add 20L of methanol and the compound 3-fluoropyridine-4-carboxylic acid (2.0kg, 14.18mol, 1.0eq), stir to dissolve evenly, and then add concentrated sulfuric acid. The mixture was warmed to 70°C and stirred overnight to check for completion.
[0047]After the mixture was brought to room temperature, it was concentrated to remove the solvent, and the concentrated solution was cooled to 0° C. and adjusted to pH=9 with saturated sodium bicarbonate solution. After extraction with ethyl acetate, the combined organic phases were dried, filtered and concentrated to obtain the product as a yellow oil (2.06kg, 94% yield): H NMR (400MHz, CDCl3): δ8.62 (d, J=Hz, 1H), 8.54(d, J=4.8Hz, 1H), 7.77(t, J=4.8Hz, 1H), 3.98(s, 3H); MS-ESI: Theoretical value (M): 155.0; Actual value: 178.1(M+ Na + ).
[0048] 2. Preparation of methyl 2-trifluorome...
Embodiment 3
[0058] 1. Preparation of methyl 2-trifluoromethyl-3-fluoro-4-pyridinecarboxylate (VI), as shown in Reaction Formula 4.
[0059]
[0060] In a 500L reactor, add 120L dichloromethane, 50L water and the compound 3-fluoro-4-pyridinecarboxylic acid methyl ester (25.0kg, 1.0eq) shown in formula (V) and stir evenly, then add 25.0L peroxy tert-butanol and 72 kg sodium trifluoromethylsulfinate (4.0 eq). The mixture was stirred at room temperature for 24 hours and monitored for completion.
[0061] The dichloromethane solvent was carefully removed at zero degrees Celsius, and the residue was carefully added to crushed ice, stirred evenly, and extracted twice with diethyl ether. After the combined organic phases were washed three times with ice water, the organic phases were dried and concentrated at low temperature, and the obtained product (VI) was directly put into the next reaction.
[0062] 2. Preparation of 2-trifluoromethyl-3-fluoro-4-pyridinecarboxylic acid and its derivativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com