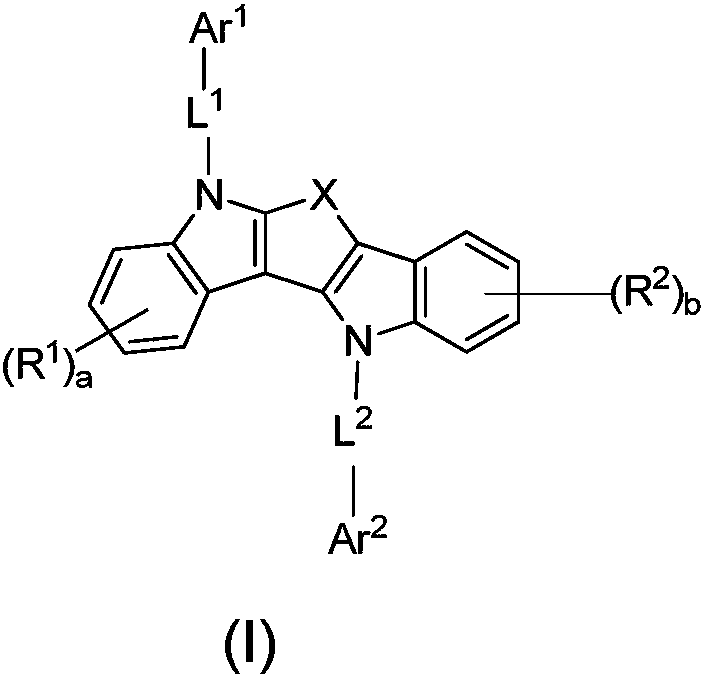
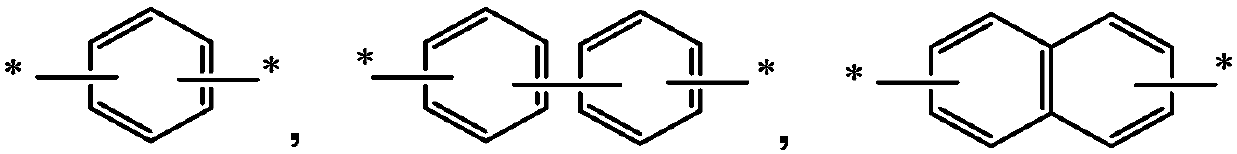
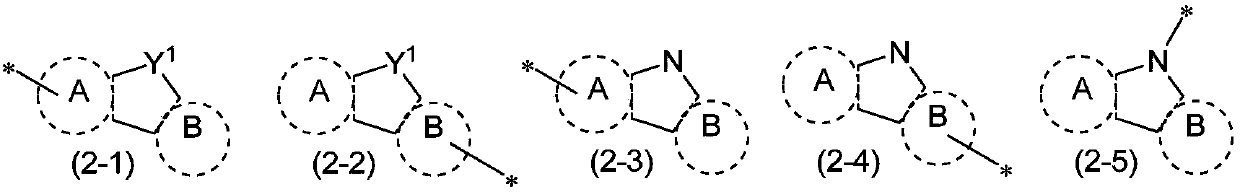
Organic electroluminescent material and applications thereof
An unsubstituted and selected technology, applied in the fields of light-emitting materials, organic chemistry, circuits, etc., can solve the problems of shortened life, mismatch of electrons and holes in the light-emitting layer, and efficiency roll-off.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 2
[0077] Synthesis of Compound P1
[0078]
[0079] In the reaction flask, add M10g (38mmol), bromobenzene 6g (38mmol), Pd 2 (dba) 3 0.1g (0.785mmol, 0.5%), S-Phos0.1g, toluene 200mL, sodium tert-butoxide 5.7, react at 100°C for 5h. After the reaction is complete, stop the reaction. Cool to room temperature, add water and ethyl acetate for extraction, and concentrate the organic phase to obtain a solid that is purified by recrystallization from toluene to obtain P1-A as a white powder.
[0080] In the reaction flask, add P1-A8g (23.7mmol), 2-bromonaphthalene 5g (23.7mmol), Pd 2 (dba) 3 0.1g (0.785mmol, 0.5%), S-Phos0.1g, xylene 200mL, sodium tert-butoxide 5.7, react at 150°C for 6h. After the reaction is complete, stop the reaction. Cool to room temperature, add water and dichloromethane for extraction, and concentrate the organic phase to obtain a solid that is purified by recrystallization from toluene to obtain P1 as a white powder.
[0081] 1 H NMR (500MHz, Chlo...
Synthetic example 3
[0083] Synthesis of compound P3
[0084] The reaction is the same as in Synthesis Example 1, except that 2-bromonaphthalene is replaced by an equivalent amount of 4-bromobiphenyl, resulting in the final product P3.
[0085] 1 H NMR (500MHz, Chloroform) δ8.55 (dd, J = 14.2, 3.7Hz, 1H), 8.00 (dd, J = 14.9, 3.0Hz, 1H), 7.92 (s, 4H), 7.81–7.69 (m, 2H),7.68–7.56(m,3H),7.56–7.35(m,7H),7.21–7.04(m,3H),6.92(td,J=15.0,3.0Hz,1H).
Synthetic example 4
[0087] Synthesis of compound P9
[0088] The reaction is the same as in Synthesis Example 1, except that 2-bromonaphthalene is replaced by an equivalent amount of 2-chloro-4,6-diphenyl-1,3,5-triazine, and the final product is P9.
[0089] 1 H NMR (500MHz, Chloroform) δ8.55 (dd, J = 14.2, 3.7Hz, 1H), 8.43–8.27 (m, 4H), 8.00 (dd, J = 14.9, 3.0Hz, 1H), 7.70–7.41 ( m,13H),7.22–7.04(m,3H),6.92(td,J=15.0,3.0Hz,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com