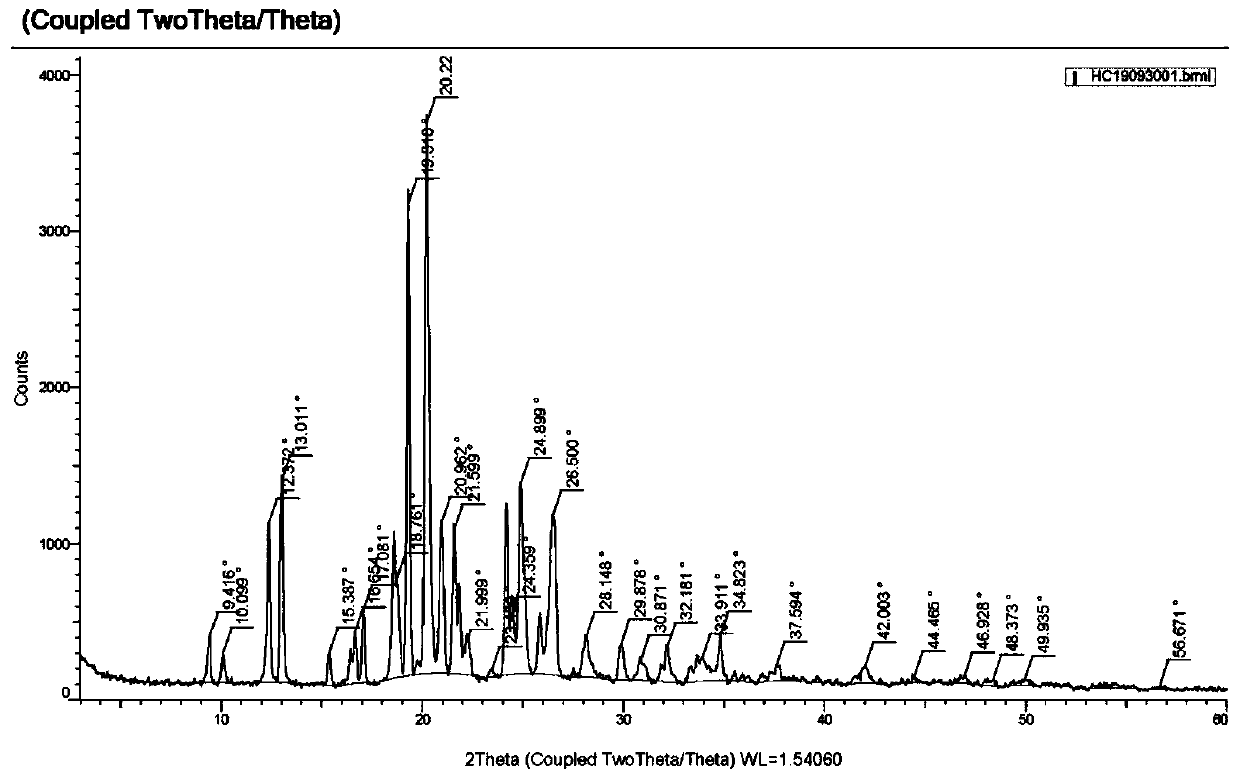
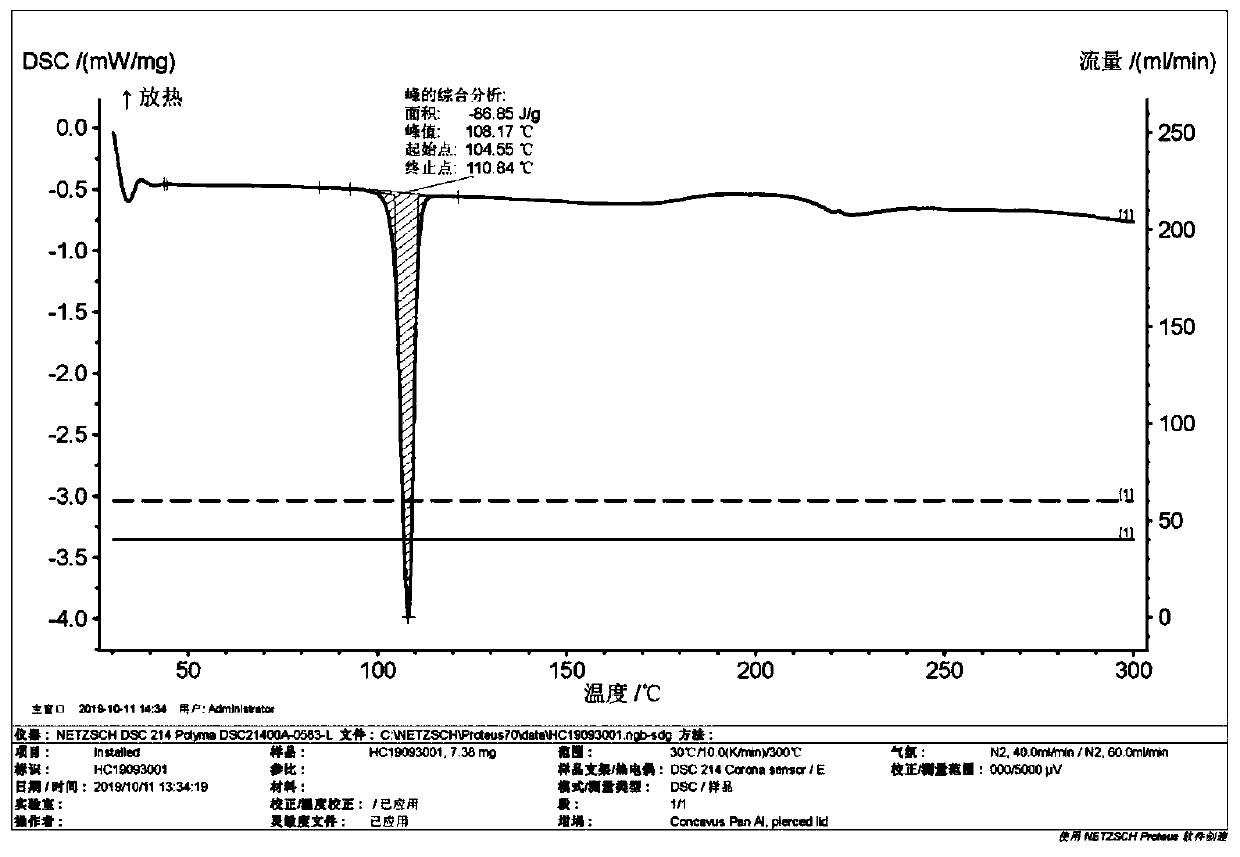
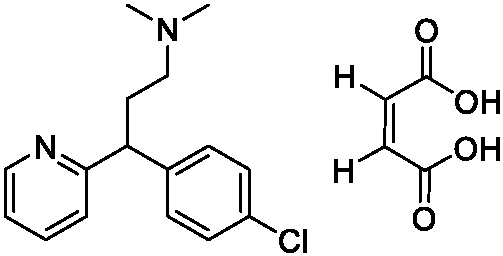
New chlorpheniramine maleate crystal and preparation method thereof
A technology of chlorpheniramine maleate and crystals, which is applied in the field of chlorpheniramine maleate crystals and its preparation, can solve the problems that affect the stability and bioavailability of original drugs and preparations, and the solubility and stability of crystal forms need to be further improved. Improve the problems of unfavorable preparation development and industrial production, and achieve the effect of improving bioavailability, good chemical stability and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Maleic acid (9.0 g, 0.078 mol) and isopropanol (22.5 g) were dissolved at 55°C, and then chlorpheniramine (22.5 g, 0.082 mol) was added to the maleic acid isopropanol solution. Subsequently, 67.5 g of methyl tert-butyl ether was added to the system for clarification. The system was lowered to 6° C. for crystallization, filtered, and the filter cake was washed with 5 g of methyl tert-butyl ether. The filter cake was dried at 35° C. to obtain chlorpheniramine maleate crystals (29 g).
Embodiment 2
[0029] Maleic acid (9 g, 0.078 mol) and isopropanol (36.0 g) were dissolved at 55° C., and then chlorpheniramine (22.5 g, 0.082 mol) was added to the maleic acid isopropanol solution. Subsequently, 150 g of methyl tert-butyl ether was added to the system for clarification, the system was lowered to 15° C. for crystallization, filtered, and the filter cake was washed with 5 g of methyl tert-butyl ether. The filter cake was dried at 45° C. to obtain chlorpheniramine maleate crystals (26 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com