Preparation method of dioctyl phthalate based on acidic eutectic solvent
A technology of dioctyl phthalate and low eutectic solvent, which is applied in the field of preparation of dioctyl phthalate, can solve the problems of many side reactions, polluted environment by acidic waste water, difficult product separation, etc., and achieves improved yield. The effect of rate and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
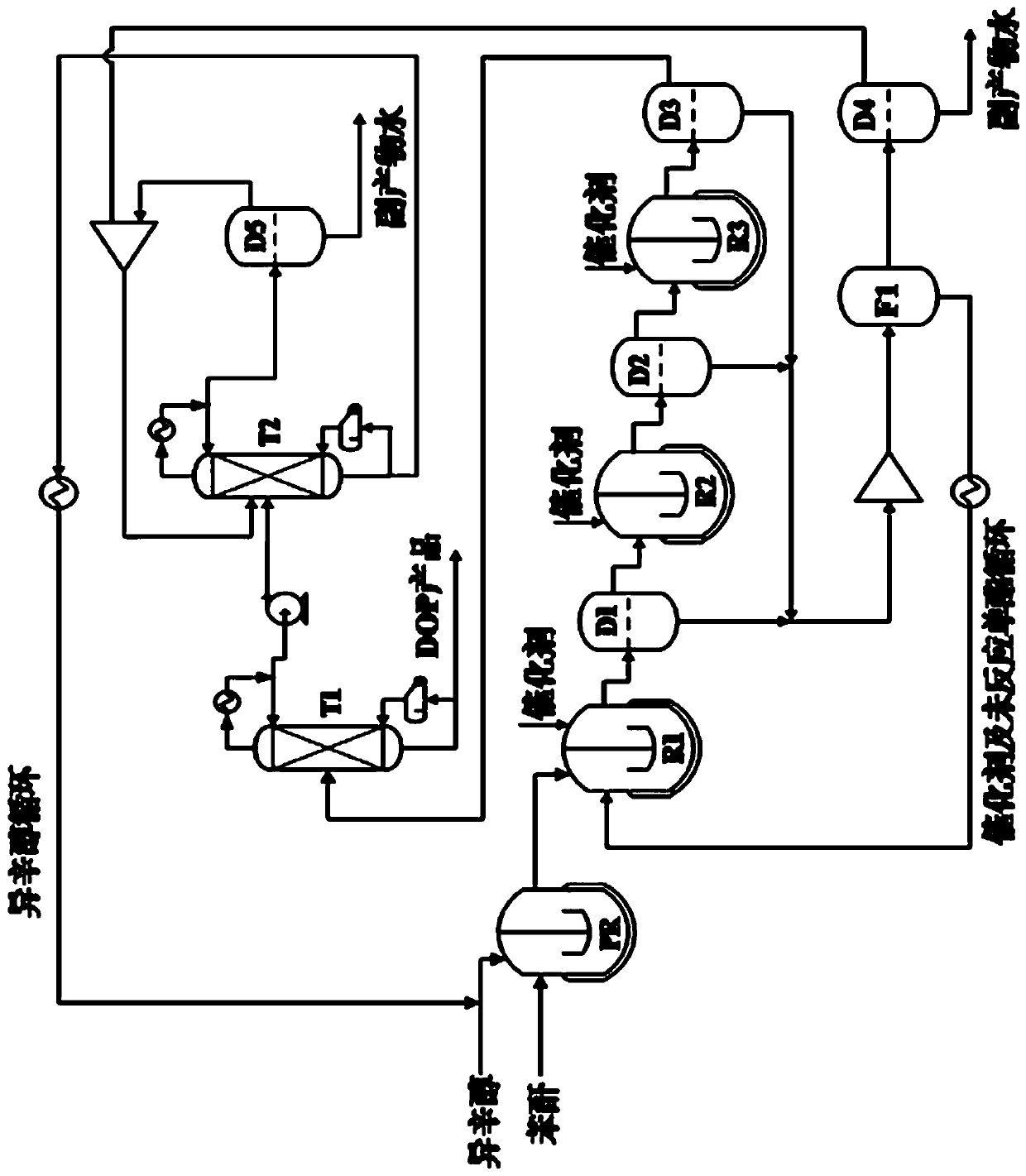
[0038] see figure 1 , the preparation method specifically includes the following steps:
[0039] S1: Carry out monoesterification with phthalic anhydride and excess isooctyl alcohol as raw materials to obtain a mixture of monoester and isooctyl alcohol; the reaction temperature of monoesterification is 80~150°C, and the reaction time is 5~120 minutes.
[0040] S2: adding a deep eutectic solvent to the mixture obtained in step S1, performing first-stage esterification, and then standing and layering to obtain an upper organic phase and a lower aqueous phase;
[0041] S3: adding a deep eutectic solvent to the organic phase obtained in step S2, performing second-stage esterification, and then standing and stratifying to obtain an upper organic phase and a lower aqueous phase;
[0042] S4: adding a deep eutectic solvent to the organic phase obtained in step S3, performing third-stage esterification, and then standing and stratifying to obtain an upper organic phase and a lower aq...
Embodiment 1
[0056] according to figure 1 The flow chart in the test is carried out, 3 kg of phthalic anhydride and 6.59 kg of isooctyl alcohol are added to the pre-reactor PR, heated to 120 ° C, stirred and reacted for 20 min under normal pressure, and then the reaction liquid enters the first-stage esterification reactor R1 , while adding 1.92kg of 2-methylimidazole-benzenesulfonic acid as a deep eutectic solvent, stirring and reacting at 120°C and normal pressure for 7 h. After the reaction, the reaction liquid was added to the decanter D1 and allowed to stand for stratification, and the standing time was 3 h. After separating the upper layer (ester phase) obtained after the phase separation in the decanter D1, it enters the second-stage esterification reactor R2, and at the same time, 1.24 kg of imidazole-p-toluenesulfonic acid deep eutectic solvent is added, at 120 ° C And continue to stir the reaction under normal pressure for 7 h. After the reaction, the reaction solution was adde...
Embodiment 2
[0061] according to figure 1 The flow chart in the test is carried out, 3 kg of phthalic anhydride and 6.59 kg of isooctyl alcohol are added to the pre-reactor PR, heated to 120 ° C, stirred and reacted under normal pressure for 20 min, and then the reaction liquid enters the first-stage esterification reactor R1, At the same time, 2.40 kg of 2-methylimidazole-benzenesulfonic acid was added as a deep eutectic solvent, and the reaction was stirred at 120° C. and normal pressure for 6.5 h. After the reaction, the reaction liquid was added to the decanter D1 and allowed to stand for stratification, and the standing time was 3 h. After separating the upper layer (ester phase) obtained after the phase separation in the decanter D1, it enters the second-stage esterification reactor R2, and at the same time, 1.64 kg of imidazole-p-toluenesulfonic acid deep eutectic solvent is added, at 120 ° C And continue stirring reaction under normal pressure for 6.5 h. After the reaction, the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com