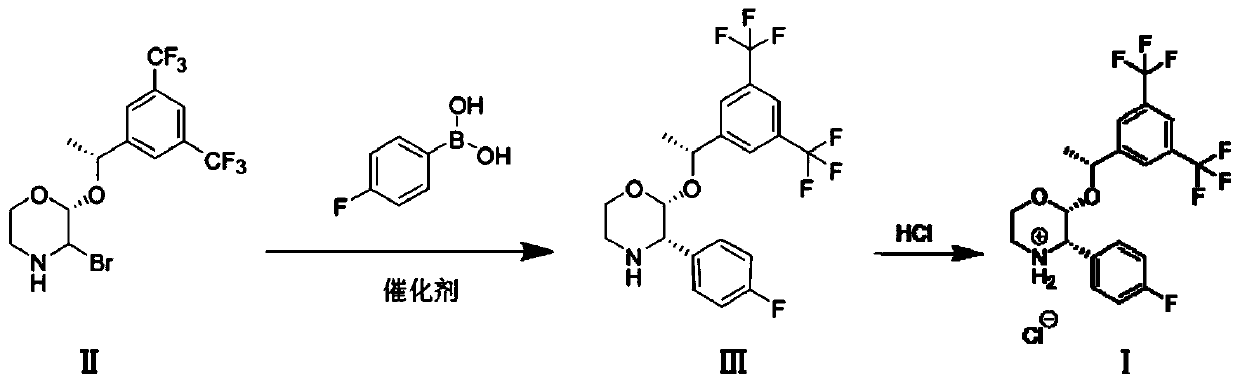
Preparation method of aprepitant intermediate
A kind of aprepitant, intermediate technology, applied in the field of chemical synthesis, can solve problems such as unfavorable production amplification, achieve the effect of less impurities, avoid potential safety hazards, and high process stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Preparation of Compound III
[0050] (1) Influence of catalyst types on the preparation of compound III
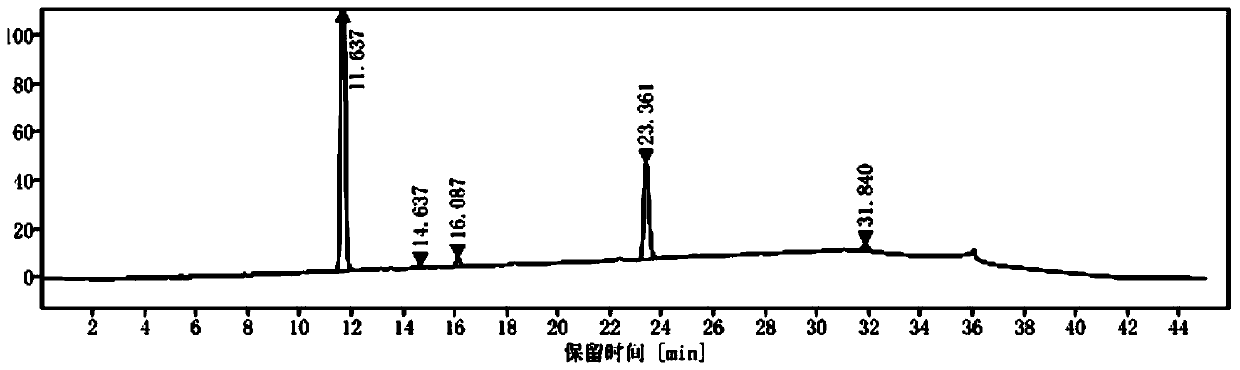
[0051] Set up experimental groups 1 to 4, respectively mix 42.2 g of compound II, 16 g of 4-fluorophenylboronic acid, 630 mL of saturated sodium carbonate aqueous solution and 1260 mL of toluene, and stir evenly, add 0.422 g of catalyst, and heat to 100 °C while stirring for 10 hours. After finishing, it was lowered to room temperature, the catalyst was recovered by filtration, the filtrate was left to stand for stratification, the upper organic phase was washed with 630 mL of saturated brine, and then liquid-separated, the organic liquid was obtained and then concentrated and recovered toluene to obtain a white blocky solid, which was the intermediate of Intermediate I. Free base——Compound Ⅲ, its high performance liquid chromatogram is as follows figure 2 shown. Table 1 shows the differences between the experimental groups 1 to 4 and the experimental ...
Embodiment 2
[0084] Example 2: Preparation of Intermediate I
[0085] (1) Influence of organic solvent types on the preparation of intermediate I
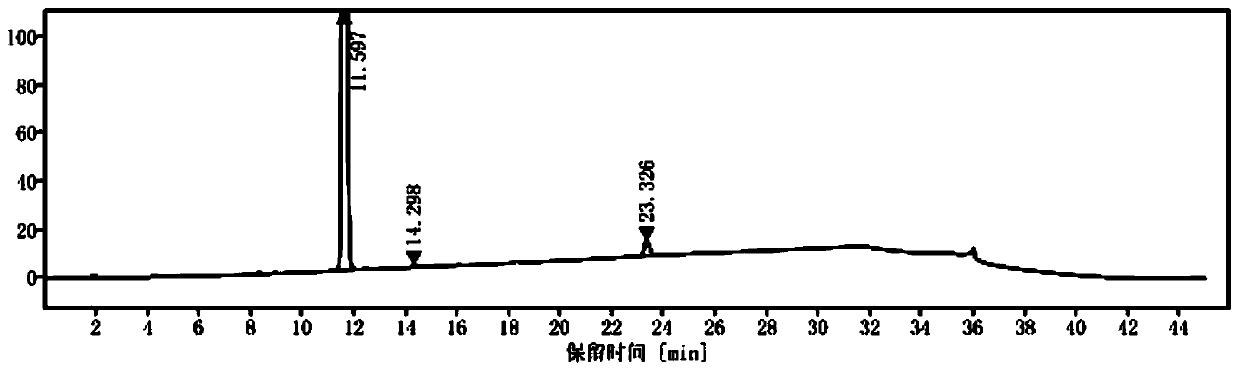
[0086] Set up experimental groups 35 to 38, respectively dissolve 44 g of compound III obtained in experimental group 1 in 660 mL of organic solvent, add hydrochloric acid with a mass concentration of 20%-38% to adjust the pH to 1-2, and then distill under reduced pressure until the volume is the original. 30%, then cooled to 0 °C to separate out a white solid, filtered and dried to obtain a white solid powder, which is Intermediate I, and its high-performance liquid chromatogram is as follows image 3 As shown, the hydrogen NMR spectrum is shown in Figure 4 shown. The differences between the experimental groups 36-39 and the experimental results are shown in Table 9.
[0087] The influence of table 9 organic solvent types on the preparation of intermediate I
[0088]
[0089] (2) the influence of methyl isobutyl ketone dosage on the pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com