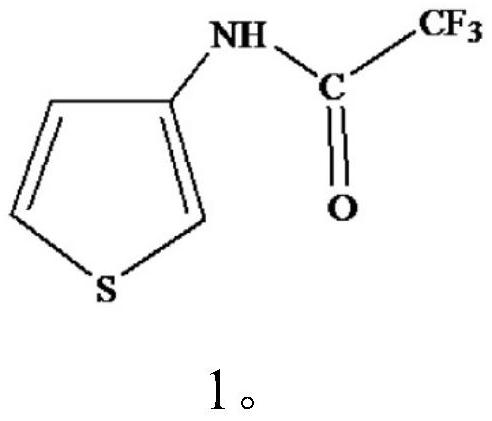
A kind of fluorine-containing thiophene derivative 3-(n-trifluoroacetylamino)thiophene and its synthesis method and application
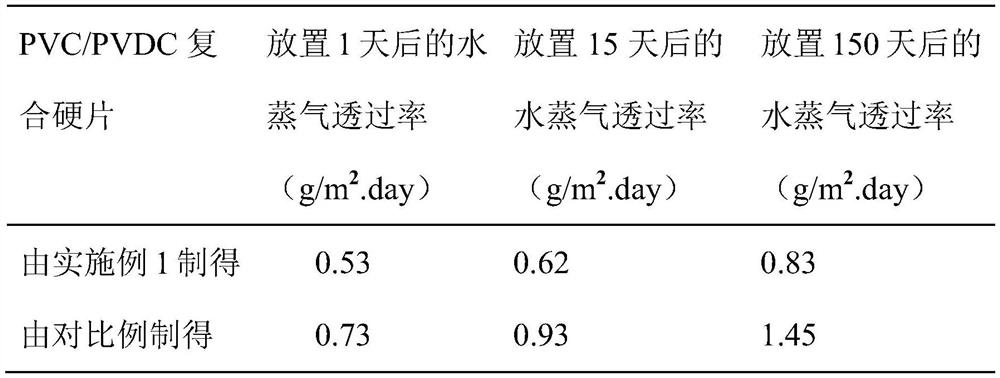
A technology of trifluoroacetamido and thiophene derivatives, used in organic chemistry, coatings, etc., can solve the problems of poor anti-ultraviolet performance, easy yellowing and aging, and achieve low water vapor transmission and anti-yellowing ability. Strong and hydrophobic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
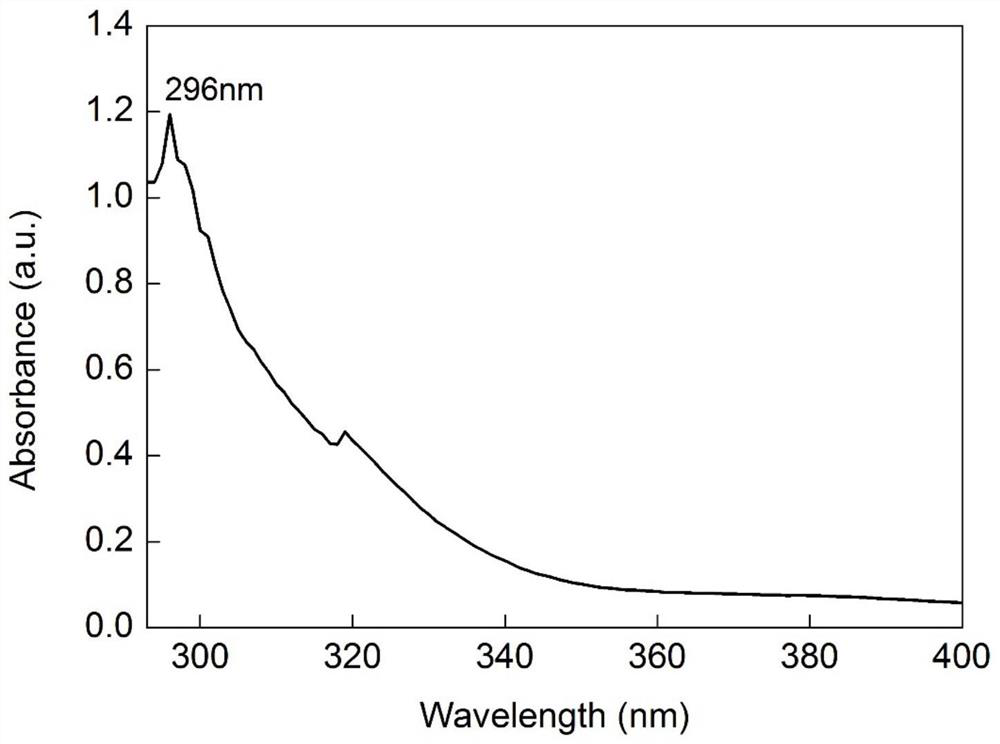
[0019] The preparation process of 3-(N-trifluoroacetylamino)thiophene of the present invention is:
[0020] 9.92g (0.1mol) 3-aminothiophene oxalate is dissolved in 128ml (2mol) methylene chloride solution, under the effect of 25.30g (0.25mol) triethylamine, obtains the 3-aminothiophene of free state, then will The reaction solution was cooled to 0°C, and 25.2 g (0.12 mol) of trifluoroacetic anhydride was slowly added dropwise. After reacting at room temperature for 2 hours, a saturated sodium bicarbonate solution was added until no bubbles were generated to obtain a mixed solution;
[0021] The mixed solution after the above reaction was extracted 3 times with ethyl acetate, and the organic phases were combined, and the organic phase was washed 3 times with saturated sodium chloride solution, dried by adding anhydrous sodium sulfate, suction filtered, and the solvent was rotary evaporated to obtain 3-( Crude N-trifluoroacetylamino)thiophene. The obtained 3-(N-trifluoroacetyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com