Pyridoxal thiosemicarbazone copper complex as well as synthesis method and application thereof
A technology of copper complexes and synthesis methods, applied in copper organic compounds, drug combinations, anti-tumor drugs, etc., can solve the problem of low anti-tumor activity, overcome the low anti-tumor activity, improve bioavailability and biological activity, The effect of improving water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
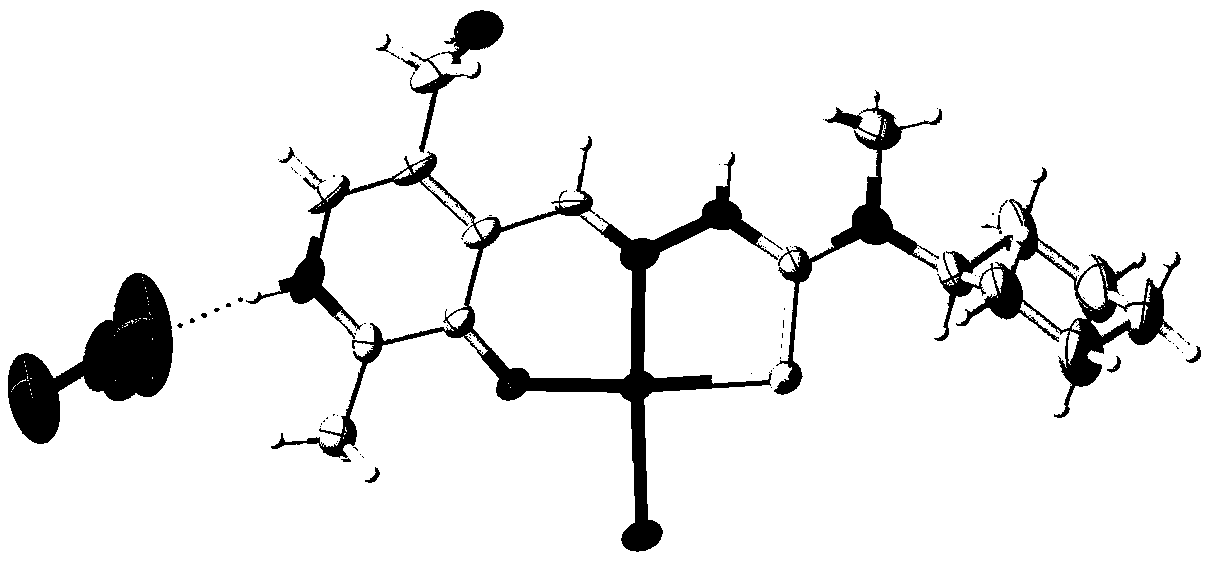
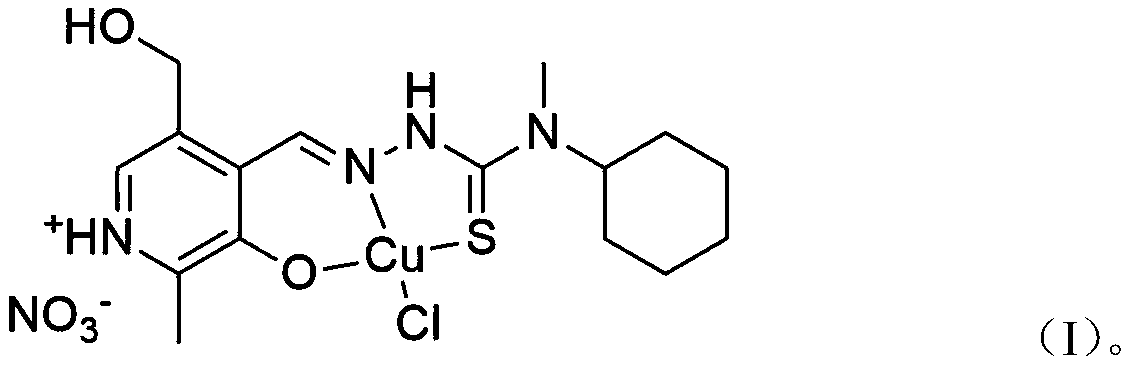
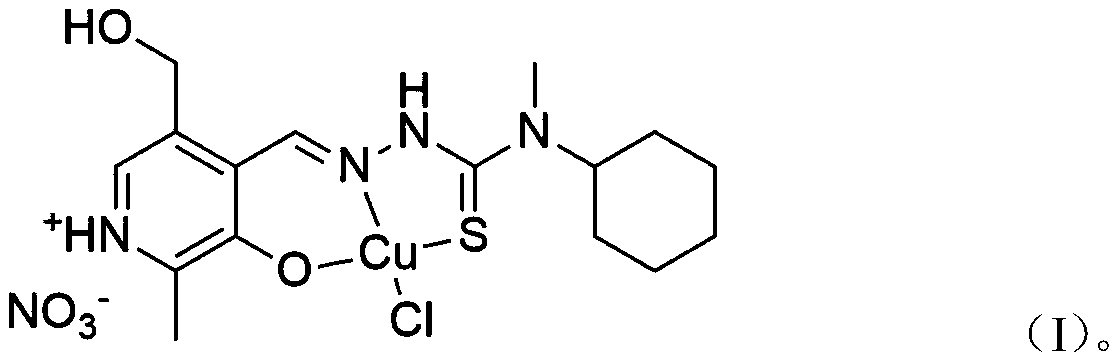
[0018] The invention provides a pyridoxal thiosemicarbazone copper complex. A kind of chlorine pyridoxal nitrate acetal 4-cyclohexyl-4-methyl-3-thiosemicarbazide copper complex, its pharmaceutically acceptable compound structure is shown in following formula (I):
[0019]
Embodiment 2
[0021] This embodiment is a synthesis method of 4-cyclohexyl-4-methyl-3-thiosemicarbazide copper complex of monochloropyridoxal nitrate, comprising the following steps:
[0022] 1) Take pyridoxal and 4-cyclohexyl-4-methyl-3-thiosemicarbazide, use alcohol as a solvent, add 1mmol hydrochloric acid dropwise, and react, and a yellow precipitate is formed;
[0023] 2) collect and react to generate precipitate, wash, obtain pyridoxal acetal 4-cyclohexyl-4-methyl-3-thiosemicarbazide hydrochloride ligand;
[0024] 3) Take the obtained ligand and dissolve it in dichloromethane, transfer it to a test tube, slowly inject the aqueous solution of copper nitrate into the test tube, so that the water phase and the dichloromethane phase are clearly separated, let it stand, and crystals are precipitated at the layered place, collect Crystals, that is, a chlorinated pyridoxal acetal 4-cyclohexyl-4-methyl-3-thiosemicarbazide copper complex.
Embodiment 3
[0026] Chlorine pyridoxal acetal 4-cyclohexyl-4-methyl-3-thiosemicarbazide copper complex synthesis method of the present embodiment is different from Example 2 in that in step 3), the ligand The ratio of the amount of substance of pyridoxal acetal 4-cyclohexyl-4-methyl-3-thiosemicarbazide and copper nitrate is that stoichiometric ratio is 1:1.2; The consumption of described solvent can dissolve the raw material that participates in reaction It is appropriate. Usually, 1 mmol of ligand pyridoxal acetal 4-cyclohexyl-4-methyl-3-thiosemicarbazide hydrochloride is dissolved with 2-10 mL of solvent; Methyl chloride, 1 mmol of ligand pyridoxal acetal 4-cyclohexyl-4-methyl-3-thiosemicarbazide or copper nitrate are dissolved in 2-10 mL of solvent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com