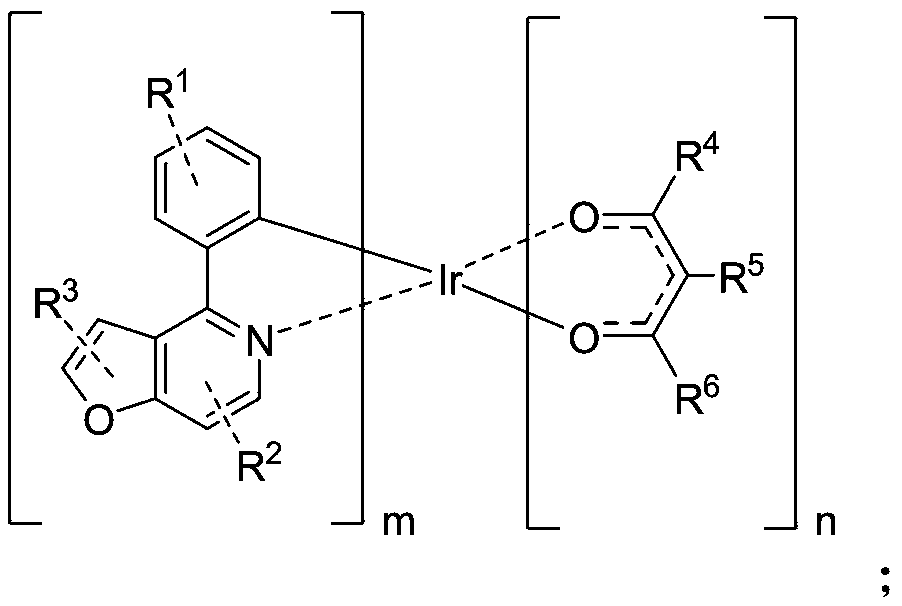
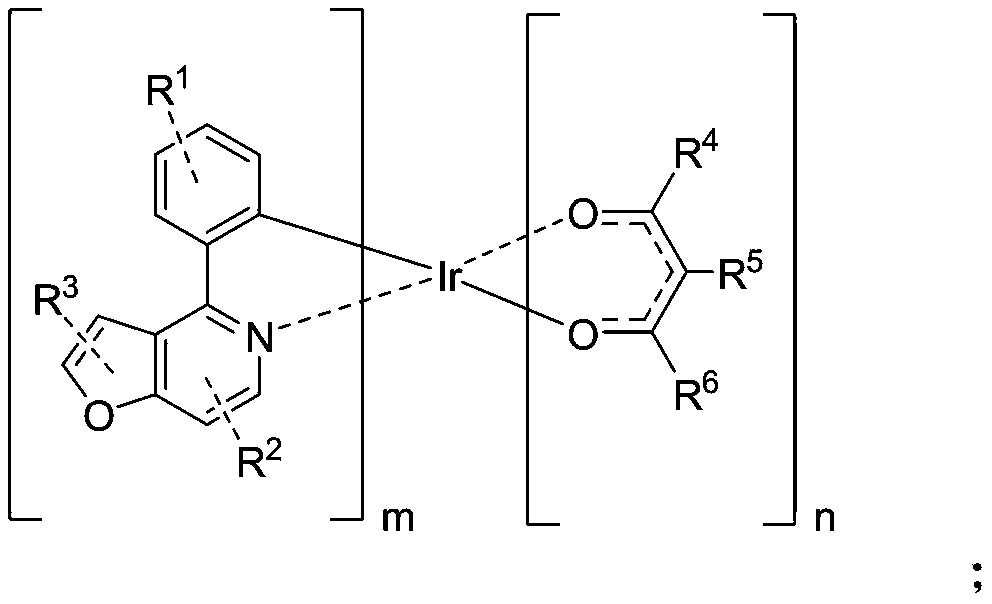
Organic phosphorescent material, preparation method thereof, and electroluminescent device containing organic phosphorescent material
A technology of optical materials and organic phosphors, applied in the field of organic phosphorescent materials and their preparation, can solve the problems of low efficiency, high voltage, low brightness, etc., and achieve the effects of high current efficiency, low driving voltage, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of Compound C-001
[0052]
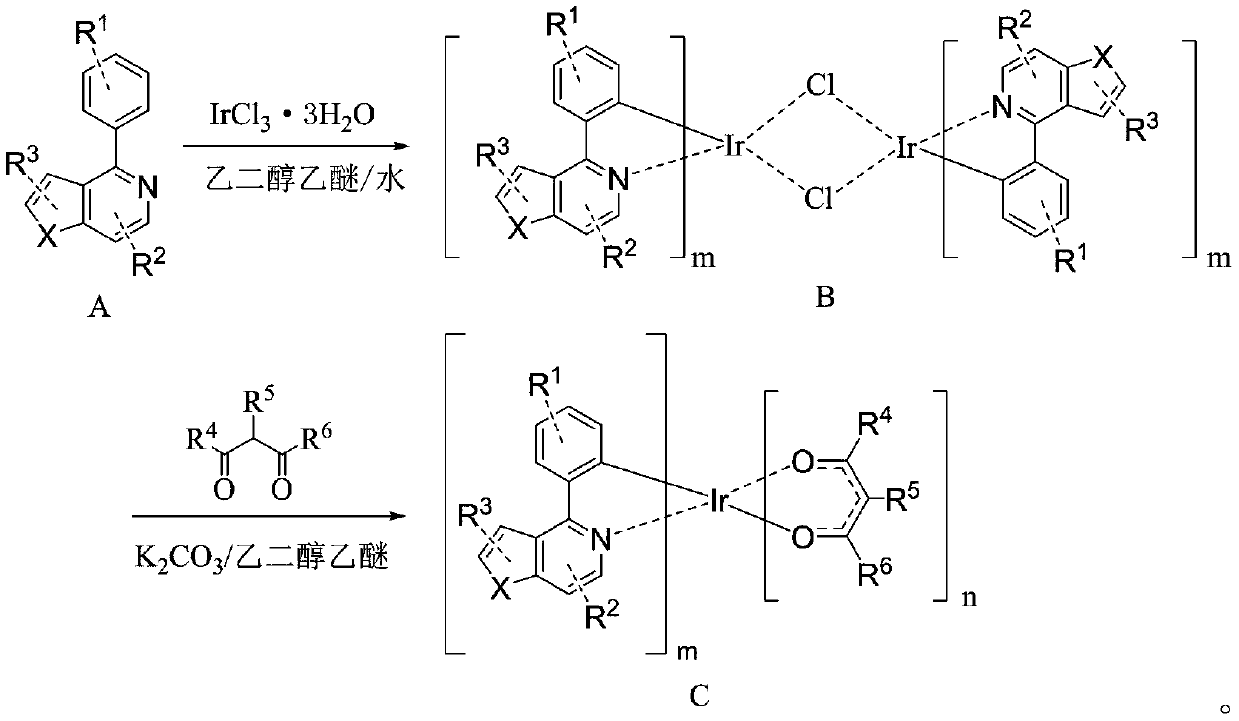
[0053] Step 1: Weigh A-001 (48mmol, 10g), IrCl 3 ·3H 2 O (19.2mmol, 6.77g), ethylene glycol ether (150ml), and water (50ml) were added to the reaction system separately. 2 Heated to reflux for 24h under protection, then cooled to room temperature, precipitated out, filtered under reduced pressure, rinsed with absolute ethanol and petroleum ether, and dried to obtain bridging ligand B-001 (6.3mmol, 8.10g) ), the yield is 65.5%;
[0054] Step 2: Weigh the bridging ligand B-001 (3.9mmol, 5g), K 2 CO 3 (26mmol, 3.60g), ethylene glycol ether (50ml) were added to the reaction system, in N 2 Add 2,4-pentanedione (11.7mmol, 1.17g) under protection, raise the temperature to 120°C, heat to reflux for 24h, cool to room temperature, filter under reduced pressure, rinse the filter cake with ethanol, and wash it at -0.1Mpa , Drying at 50° C., passing through a silica gel column, and finally spin-drying the obtained filtrate to obtain the ...
Embodiment 2
[0060] Example 2 Preparation of C-004
[0061]
[0062] Step 1: Weigh A-004 (45mmol, 10g), IrCl 3 ·3H 2 O (18mmol, 6.35g), ethylene glycol ether (150ml), and water (50ml) were added to the reaction system separately. 2 Heat to reflux for 24 hours under protection, and then cool to room temperature. Precipitate precipitates. Perform vacuum filtration, rinse with absolute ethanol and petroleum ether, and dry to obtain bridging ligand B-004 (5.8mmol, 7.80g ), the yield is 64.4%;
[0063] Step 2: Weigh the bridging ligand B-004 (3.7mmol, 5g), K 2 CO 3 (26mmol, 3.60g), ethylene glycol ether (50ml) were added to the reaction system, in N 2 Add 2,4-pentanedione (11.1mmol, 1.11g) under protection, raise the temperature to 120°C, heat to reflux for 24h, cool to room temperature, filter under reduced pressure, rinse the filter cake with ethanol, and wash it at -0.1Mpa , Dried at 50°C, passed through a silica gel column, and finally spin-dried the obtained filtrate to obtain the target produc...
Embodiment 3
[0069] Example 3 Preparation of C-007
[0070]
[0071] Step 1: Weigh A-007 (42mmol, 10g), IrCl 3 ·3H 2 O (16.8mmol, 5.92g), ethylene glycol ether (150ml), and water (50ml) were added to the reaction system separately. 2 Heated to reflux for 24h under protection, then cooled to room temperature, precipitated out, filtered under reduced pressure, rinsed with absolute ethanol and petroleum ether, and dried to obtain bridging ligand B-007 (5.1mmol, 7.10g ), the yield is 60.3%;
[0072] Step 2: Weigh the bridging ligand B-007 (3.6mmol, 5g), K 2 CO 3 (26mmol, 3.60g), ethylene glycol ether (50ml) were added to the reaction system, in N 2 Add 2,4-pentanedione (10.8mmol, 1.08g) under protection, raise the temperature to 120°C, heat to reflux for 24h, cool to room temperature, filter under reduced pressure, rinse the filter cake with ethanol, and wash it at -0.1Mpa , Drying at 50° C., passing through a silica gel column, and finally spin-drying the obtained filtrate to obtain the target pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com