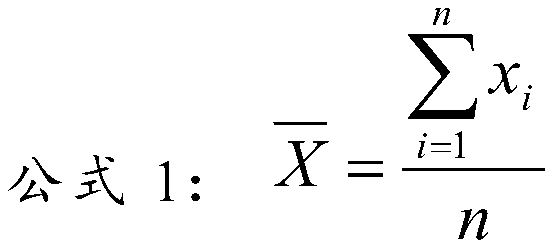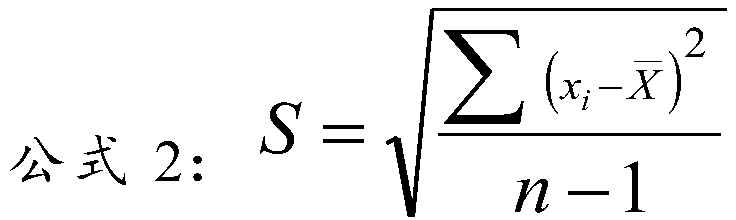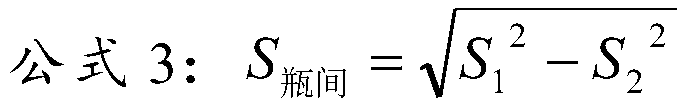Lipid quality control product and preparation method thereof
A technology for quality control substances and lipids, applied in the field of clinical medical testing, can solve the problems of matrix effects, inconvenient quality control, and incomplete inclusion of items, and achieve the effects of stable channel sources, wide application range, and reduced matrix effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: lipid quality control product of the present invention
[0031] The matrix solution is composed of the following components: normal human serum, 50mmol / LHEPES buffer, 50g / L trehalose, 0.2%NaN 3 ;
[0032] The content of the lipid component:
[0033] 1) The content of each lipid component in the high-level lipid quality control product is total cholesterol (TC) 8.0mmol / L, triglyceride (TG) 4.0mmol / L, high-density lipoprotein cholesterol (HDL-C) 1.6mmol / L, low-density lipoprotein cholesterol (LDL-C) 5.0mmol / L, apolipoprotein A1 (ApoA-1) 2.5g / L, apolipoprotein B (ApoB) 1.6g / L, lipoprotein a( Lp(a)) 300mg / L, small and dense low-density lipoprotein cholesterol (sd LDL-C) 2.5mmol / L;
[0034] 2) The content of each lipid component in the middle-level lipid quality control product is: total cholesterol (TC) 5.5mmol / L, triglyceride (TG) 2.5mmol / L, high-density lipoprotein cholesterol (HDL-C ) 1.3mmol / L, low-density lipoprotein cholesterol (LDL-C) 3.5mmol / L, apo...
Embodiment 2
[0036] Embodiment 2: lipid quality control product of the present invention
[0037] The matrix solution is composed of the following components: normal human serum, 25mmol / L HEPES buffer, 100g / L sucrose, 0.15% Proclin300;
[0038] The content of the lipid component:
[0039] 1) The content of each lipid component in the high-level lipid quality control product is total cholesterol (TC) 8.3mmol / L, triglyceride (TG) 4.5mmol / L, high-density lipoprotein cholesterol (HDL-C) 1.5mmol / L, low-density lipoprotein cholesterol (LDL-C) 4.8mmol / L, apolipoprotein A1 (ApoA-1) 2.3g / L, apolipoprotein B (ApoB) 1.5g / L, lipoprotein a ( Lp(a)) 280mg / L, small and dense low-density lipoprotein cholesterol (sd LDL-C) 2.4mmol / L;
[0040] 2) The content of each lipid component in the middle-level lipid quality control product is: total cholesterol (TC) 5.8mmol / L, triglyceride (TG) 2.6mmol / L, high-density lipoprotein cholesterol (HDL-C ) 1.3mmol / L, low-density lipoprotein cholesterol (LDL-C) 3.6mmol / ...
Embodiment 3
[0042] Embodiment 3: lipid quality control product of the present invention
[0043] The matrix solution is composed of the following components: normal human serum, 75mmol / LHEPES buffer, 50g / L pentaerythritol, 0.2%NaN 3 ;
[0044] The content of the lipid component:
[0045] 1) The content of each lipid component in the high-level lipid quality control product is total cholesterol (TC) 7.5mmol / L, triglyceride (TG) 4.2mmol / L, high-density lipoprotein cholesterol (HDL-C) 1.5mmol / L, low-density lipoprotein cholesterol (LDL-C) 4.5mmol / L, apolipoprotein A1 (ApoA-1) 2.2g / L, apolipoprotein B (ApoB) 1.5g / L, lipoprotein a ( Lp(a)) 310mg / L, small and dense low-density lipoprotein cholesterol (sd LDL-C) 2.3mmol / L;
[0046] 2) The content of each lipid component in the middle-level lipid quality control product is: total cholesterol (TC) 5.5mmol / L, triglyceride (TG) 2.5mmol / L, high-density lipoprotein cholesterol (HDL-C ) 1.1mmol / L, low-density lipoprotein cholesterol (LDL-C) 3.2mmol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com