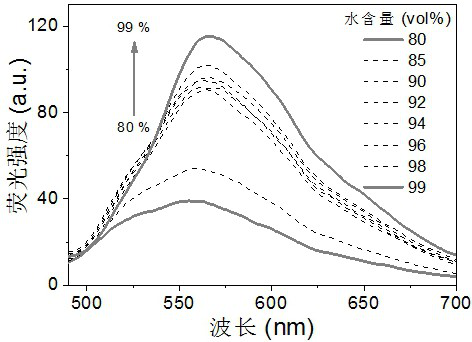
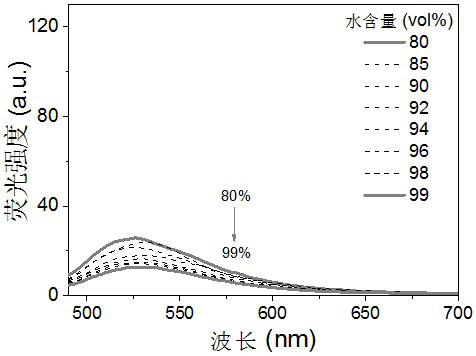
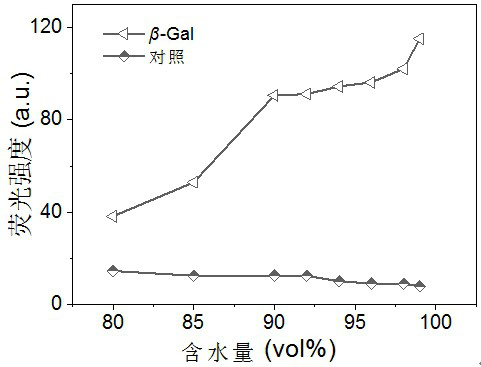
Cyanovinylidene derivative fluorescent dye and its preparation method and application
A technology of fluorescence imaging and acetonitrile, applied in the field of chemical sensors, can solve the problems of photophysical properties restricting development and application, and achieve remarkable results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] (1) Weigh 2-benzimidazole acetonitrile (266.0 mg, 1.70 mmol) and 4-(N,N-diethyl)aminobenzaldehyde to dissolve in ethanol (20 mL), then add piperidine (1.0 mL, 17.0 mmol), stirred at room temperature for 4h under the protection of an inert gas. After the reaction, a large amount of homogeneous orange-yellow fine sand-like precipitates were formed in the solution. The reaction solution was filtered, washed with ice ethanol, and the filter residue was dried to obtain an orange-yellow powder solid, which was the relatively pure product 3 (396.0 mg, 72%); 1 H NMR (400 MHz, DMSO-d 6 ): δ = 12.79 (s, 1H), 8.11 (s, 1H), 7.90 (d, 2H, J = 8.4 Hz),7.63 (d, 1H, J = 7.2 Hz), 7.50 (d, 1H, J = 7.2 Hz), 7.20 (m, 2H, J = 7.2 Hz),6.84 (d, 2H, J = 8.4 Hz), 3.46 (q, 4H, J = 7.2 Hz), 1.14 (t, 6H, J = 6.4 Hz); 13 C NMR (100 MHz, DMSO-d 6 ): δ = 150.6, 149.5, 145.8, 144.0, 135.3, 132.8, 123.2, 122.3, 119.6, 119.0, 118.4, 111.8, 111.6, 93.4, 44.4, 12.9; HRMS ...
Embodiment 2
[0038] Example 2 Preparation of Compound 12
[0039] The preparation method of compound 3 is the same as that in Example 1.
[0040]
[0041] (1) Weigh p-hydroxybenzaldehyde 6 (257.0 mg, 2.10 mmol) and dissolve it in 1 mol / L sodium hydroxide solution,
[0042] 2, 3, 4, 6-tetraacetyl- β -D-Glucopyranose 7 (867.0 mg, 2.10 mmol), the reaction was stirred at reflux (60 ± 3 °C) for 4 h. When the solution turned yellow completely, the solvent was removed by distillation under reduced pressure after the reaction. The obtained solid was subjected to silica gel column chromatography (eluent: ethyl acetate / petroleum ether = 1 / 3) to obtain compound 8 (598.0 mg, 63%); 1 H NMR (400 MHz, CDCl 3 ) : δ = 9.92 (s, 1H), 7.86 (d, 2H, J =8.8 Hz), 7.12 (d, 2H, J = 8.0 Hz), 5.51 (d, 2H, J = 17.6 Hz), 5.20 (d, 1H, J = 8.0 Hz), 5.15 (d, 1H, J = 9.6 Hz), 4.16 (m, 3H), 2.195 (s, 3H), 2.07 (s,6H), 2.026 (s, 3H). HRMS (ESI): calcd for C 21 h 25 o 11 [M+H] + m / z 452.1319, found 4...
Embodiment 3
[0053] Using a method similar to Example 2, those skilled in the art can easily prepare R 1 independently selected from NH 2 , OH, CN, CH 3 , COOH, SO 3 H, F, Cl, Br or NO 2 , R 2 for compound of.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com