Detection method of 2-(thiophene-2-yl)ethyl p-toluenesulfonate and isomers thereof
A technology of p-toluenesulfonate and a detection method is applied in the field of detection of 2-ethyl p-toluenesulfonate and its isomers, which can solve the problems of affecting the purity and quality of raw materials, being difficult to separate, and the like, and achieve accuracy High, easy to operate, good sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
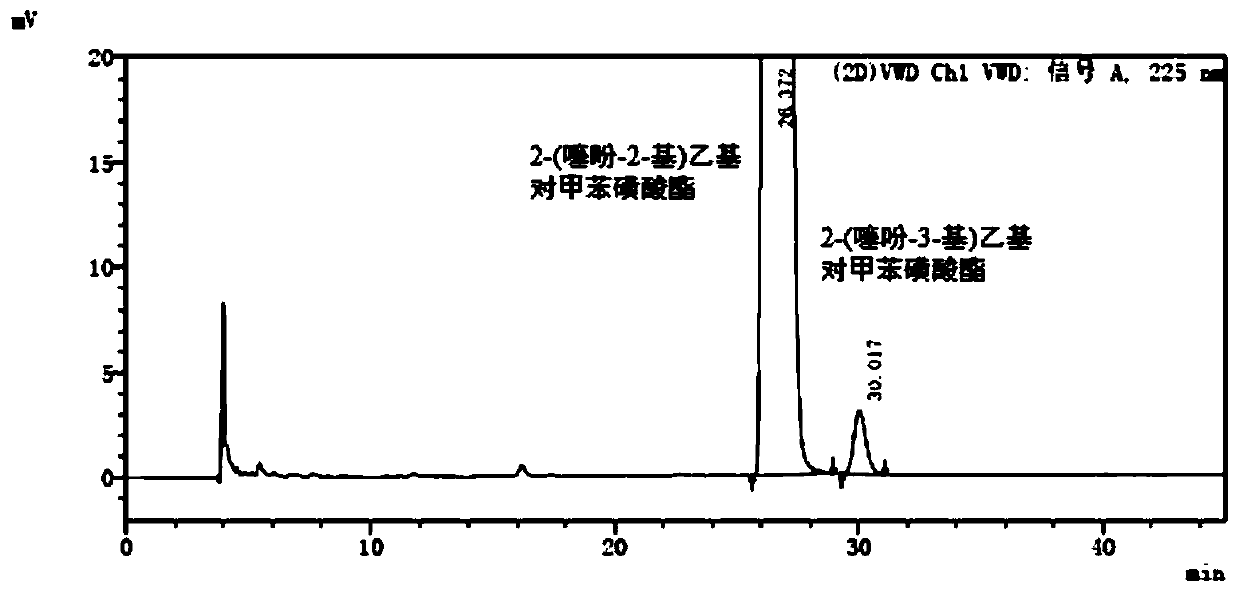
[0057] A detection method for 2-(thiophen-2-yl)ethyl p-toluenesulfonate and its isomers, comprising:
[0058] Step S1, dissolving 2-(thiophen-2-yl)ethyl p-toluenesulfonate in n-hexane to make a supply containing 1mg of 2-(thiophen-2-yl)ethyl p-toluenesulfonate per 1mL Test solution; 2-(thiophen-3-yl) ethyl p-toluenesulfonate was dissolved in n-hexane to make a solution containing 2 μg of 2-(thiophen-3-yl) ethyl p-toluenesulfonate per 1 mL Reference substance solution.
[0059] Step S2, dissolve 2-(thiophen-2-yl)ethyl p-toluenesulfonate and 2-(thiophen-3-yl)ethyl p-toluenesulfonate reference substance in n-hexane to prepare each 1mL containing 2 -(thiophen-2-yl)ethyl p-toluenesulfonate 1mg and 2-(thiophen-3-yl)ethyl p-toluenesulfonate 2μg system suitability solution.
[0060] In step S3, the test solution, the reference solution and the system suitability solution are subjected to normal-phase high-performance liquid chromatography detection. The instrument is an Agilent 1260...
Embodiment 2
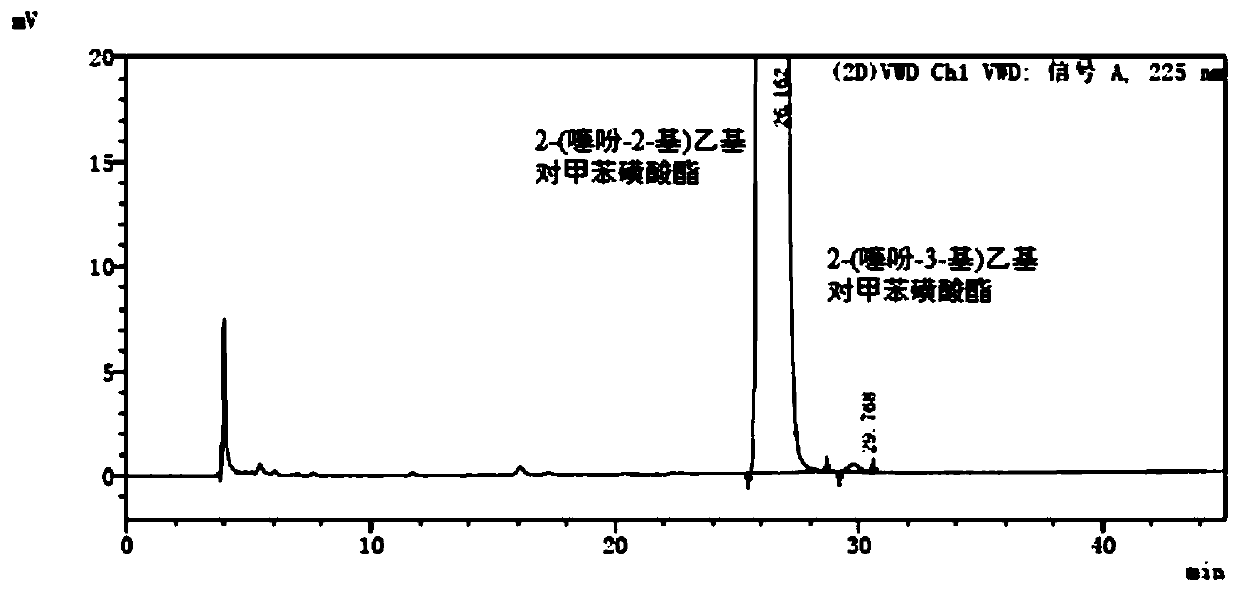
[0065] A detection method for 2-(thiophen-2-yl)ethyl p-toluenesulfonate and its isomers, comprising:
[0066]Step S1, dissolving 2-(thiophen-2-yl)ethyl p-toluenesulfonate in n-hexane to make a supply containing 1mg of 2-(thiophen-2-yl)ethyl p-toluenesulfonate per 1mL Test solution; 2-(thiophen-3-yl) ethyl p-toluenesulfonate was dissolved in n-hexane to make a solution containing 2 μg of 2-(thiophen-3-yl) ethyl p-toluenesulfonate per 1 mL Reference substance solution.
[0067] Step S2, dissolve 2-(thiophen-2-yl)ethyl p-toluenesulfonate and 2-(thiophen-3-yl)ethyl p-toluenesulfonate reference substance in n-hexane to prepare each 1mL containing 2 -(thiophen-2-yl)ethyl p-toluenesulfonate 1mg and 2-(thiophen-3-yl)ethyl p-toluenesulfonate 2μg system suitability solution.
[0068] Step S3, the test solution, the reference solution and the system suitability solution are subjected to normal phase high performance liquid chromatography detection, the instrument is a Waters 2695-996 l...
Embodiment 3
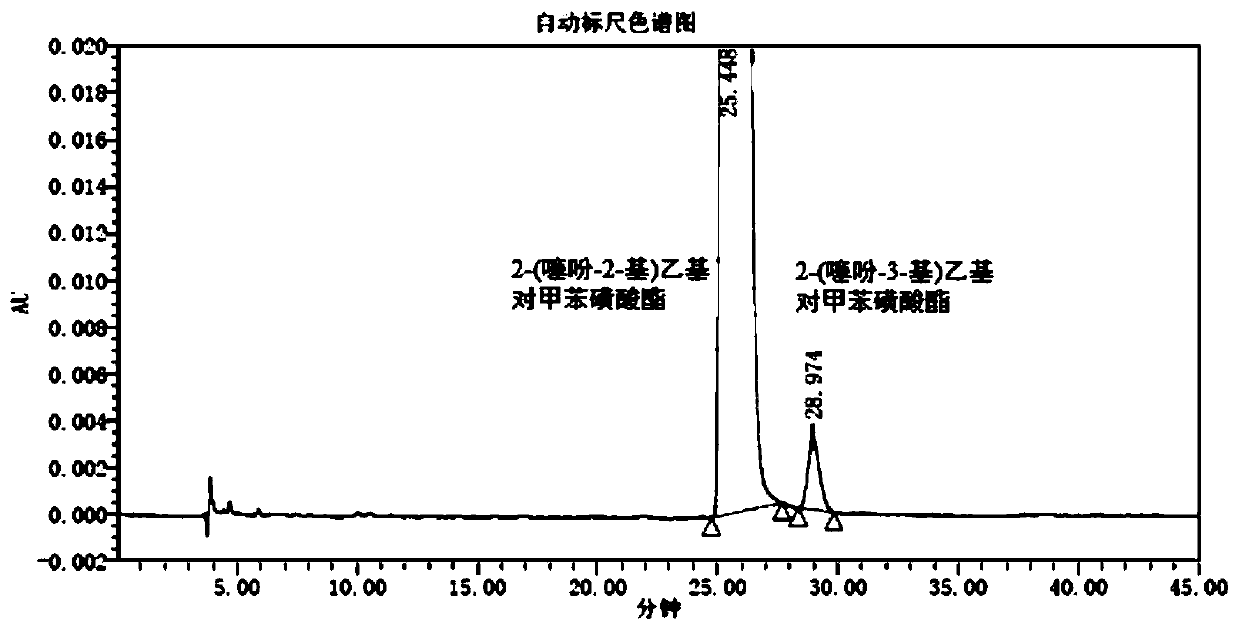
[0073] A detection method for 2-(thiophen-2-yl)ethyl p-toluenesulfonate and its isomers, comprising:
[0074] Step S1, dissolving 2-(thiophen-2-yl)ethyl p-toluenesulfonate in n-hexane-absolute ethanol (50:50) to make every 1mL containing 2-(thiophen-2-yl)ethyl The test solution of 1.5 mg of ethyl p-toluenesulfonate; 2-(thiophen-3-yl) ethyl p-toluenesulfonate was dissolved in n-hexane-absolute ethanol (50:50) to make every 1mL A reference solution containing 10 μg of 2-(thiophen-3-yl)ethyl p-toluenesulfonate.
[0075] Step S2, dissolve 2-(thiophen-2-yl)ethyl p-toluenesulfonate and 2-(thiophen-3-yl)ethyl p-toluenesulfonate reference substance in n-hexane-absolute ethanol (50: 50), prepare each 1mL containing 2-(thiophen-2-yl)ethyl p-toluenesulfonate 1.5mg and 2-(thiophen-3-yl)ethyl p-toluenesulfonate and other known impurities 10 μg of system suitability solution.
[0076] Step S3, the test solution, the reference solution and the system suitability solution are subjected to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com