Method for preparing (S)-2-(3,4-difluorophenyl) oxirane
A technology of difluorophenyl and ethylene oxide, which is applied in the field of one-step enzymatic preparation of ticagrelor intermediate-2-oxirane, can solve problems such as being unsuitable for industrialized generation and application, and achieve mild reaction conditions, Simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of Ketoreductase
[0030] The genetically engineered bacteria (vector pET21a, host cell E.Coli BL21(DE3)) containing the coding gene of ketoreductase (SEQ ID No.1) was inoculated into 5mL LB test tube medium containing ampicillin for activation culture (37°C) Cultivate for 12h), transfer the activated culture to 400mL LB liquid medium containing ampicillin according to 1% inoculum, cultivate OD to 0.6-0.8 at 37°C, add IPTG (final concentration 0.1mM) and induce culture at 25°C for 16h. The cells were collected by centrifugation to obtain ketoreductase cells. After resuspending the cells in 40mL phosphate buffer (10mM, pH 7.5), the cells were sonicated for 15min in an ice water bath, and the supernatant was collected by centrifugation, pre-frozen at -20°C and then vacuum freeze-dried After 48h, crushed to obtain recombinant ketoreductase enzyme powder.
Embodiment 2
[0031] Example 2 Preparation of (S)-2-(3,4-difluorophenyl) ethylene oxide
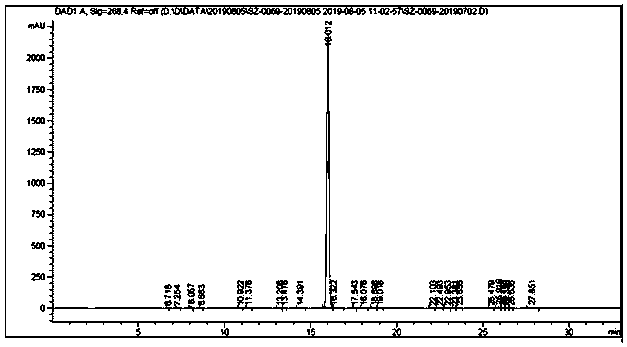
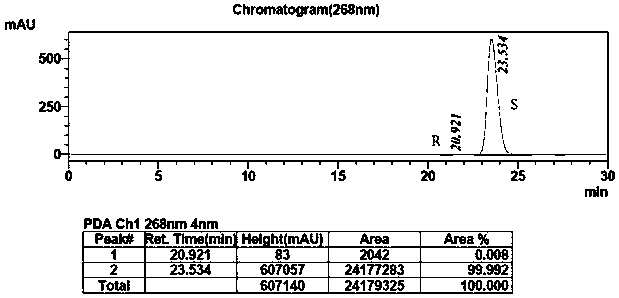
[0032] Add methyl tert-butyl ether (500mL), 0.05M pH=9.0 phosphate buffer (300mL), isopropanol (100g) and the substrate 2-chloro-1-(3,4-di Fluorophenyl) ethyl ketone (200g), after stirring evenly, add ketoreductase enzyme powder (20g), coenzyme NADP (60mg) and D201 resin, and react with magnetic stirring at 25°C for 12h while TLC monitors the progress of the reaction. After the reaction, the filtrate was filtered, the filtrate was allowed to stand for separation, and the organic phase was collected. The aqueous phase was extracted twice with 100 mL*2 methyl tert-butyl ether, combined with the aforementioned organic phase, dried over anhydrous sodium sulfate, and spin-dried under reduced pressure. 155.7 g of (S)-2-(3,4-difluorophenyl) ethylene oxide was obtained, and the ee value of the S type product was 99.99%. The infrared spectrum of the product is attached figure 1 , The product purity HPLC spectrum ...
Embodiment 3
[0033] Example 3 Preparation of (S)-2-(3,4-difluorophenyl) ethylene oxide
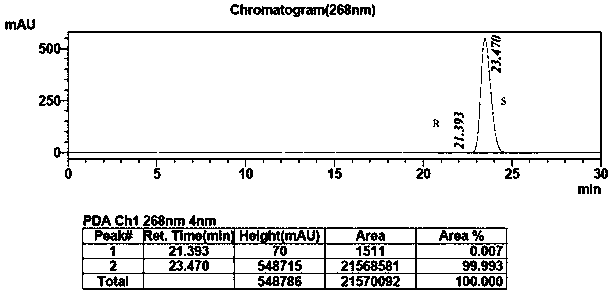
[0034] Add methyl tert-butyl ether (2.5L), 0.05M pH=10.0 phosphate buffer (2.5L), isopropanol (1kg) and the substrate 2-chloro-1-(3,4) to the reaction vessel -Difluorophenyl) ethyl ketone (2kg), after stirring evenly, add ketoreductase enzyme powder (500g), coenzyme NADP (600mg) and D201 resin, and magnetically stir the reaction at 25°C for 20h while TLC monitors the progress of the reaction. After the reaction, the filtrate was filtered, the filtrate was allowed to stand for separation, and the organic phase was collected. The aqueous phase was extracted twice with 100 mL*2 methyl tert-butyl ether, combined with the aforementioned organic phase, dried over anhydrous sodium sulfate, and spin-dried under reduced pressure. 1.52 kg of (S)-2-(3,4-difluorophenyl) ethylene oxide was obtained, and the ee value of the S type product was 99.99%. The chiral purity HPLC spectrum of the product is attached Figure 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com