Kit for detecting polymorphism of hypertension medication related genes
A gene polymorphism and kit technology, which is applied in the determination/inspection of microorganisms, biochemical equipment and methods, etc., to achieve the effects of low cost, simple and feasible interpretation method, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1: the composition of kit
[0105] The composition of kit of the present invention is as follows:
[0106] Table 2 Composition of the kit
[0107]
[0108] Table 3 Primers and Probes
[0109]
[0110]
Embodiment 2
[0111] Embodiment 2: Extraction of sample genomic DNA
[0112] In this example, human whole blood / peripheral blood samples were collected, and genomic DNA was extracted therefrom.
[0113] Genomic DNA was extracted from EDTA anticoagulated plasma and used as a template for PCR detection. Use pretreatment reagents and nucleic acid extraction and purification reagents (magnetic bead method) from Zhengzhou Antu Bioengineering Co., Ltd., and follow the instructions. The details are as follows:
[0114] 1. Sample pretreatment:
[0115] 1) Pretreatment of frozen-thawed whole blood with red blood cell lysate:
[0116] ①Take 200 μL clinical frozen-thawed or hemolyzed whole blood sample, add 600 μL red blood cell lysate, and gently invert and mix 10 times. After the red blood cell is lysed, the solution should be clear and transparent. 12000rpm / min, centrifuge for 3min, discard the supernatant;
[0117] ②Add 600μL 0.2mg / ml proteinase K, incubate at room temperature 20-25℃ for 5min,...
Embodiment 3
[0129] Embodiment 3: the detection of sample genomic DNA
[0130] In this example, the combination of primers and probes listed in Example 1 is used to amplify the genomic DNA sample extracted in Example 2. The detection steps are as follows:
[0131] ① Build a reaction system
[0132] Calculate the number of samples required, increase the amount of reagents in proportion to the amount in the table below, and only do one copy of negative and positive quality control.
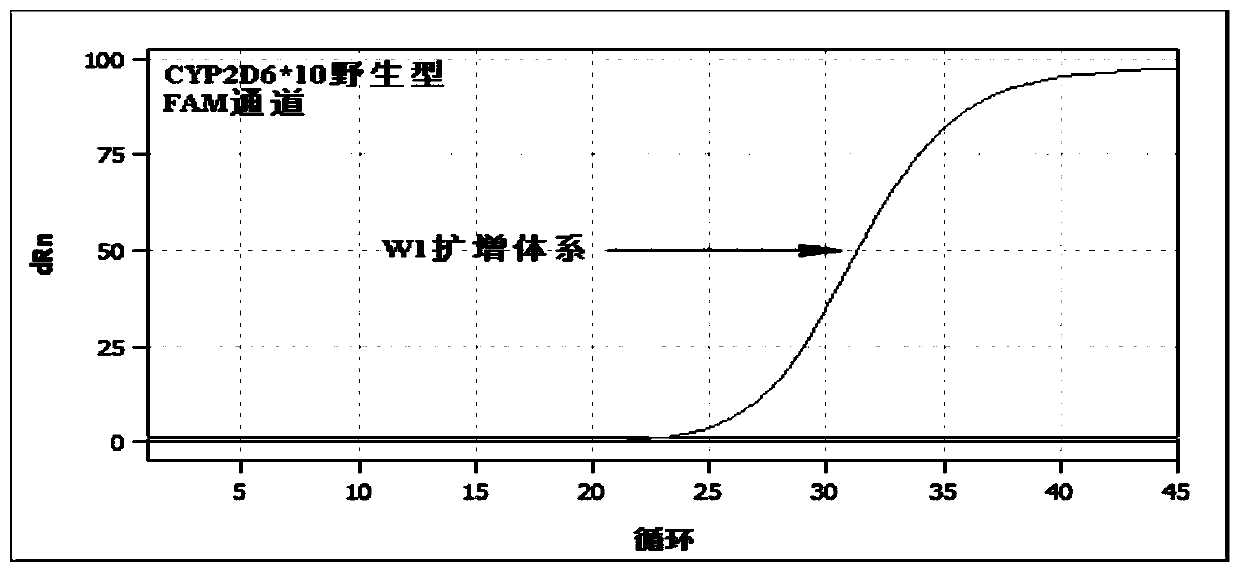
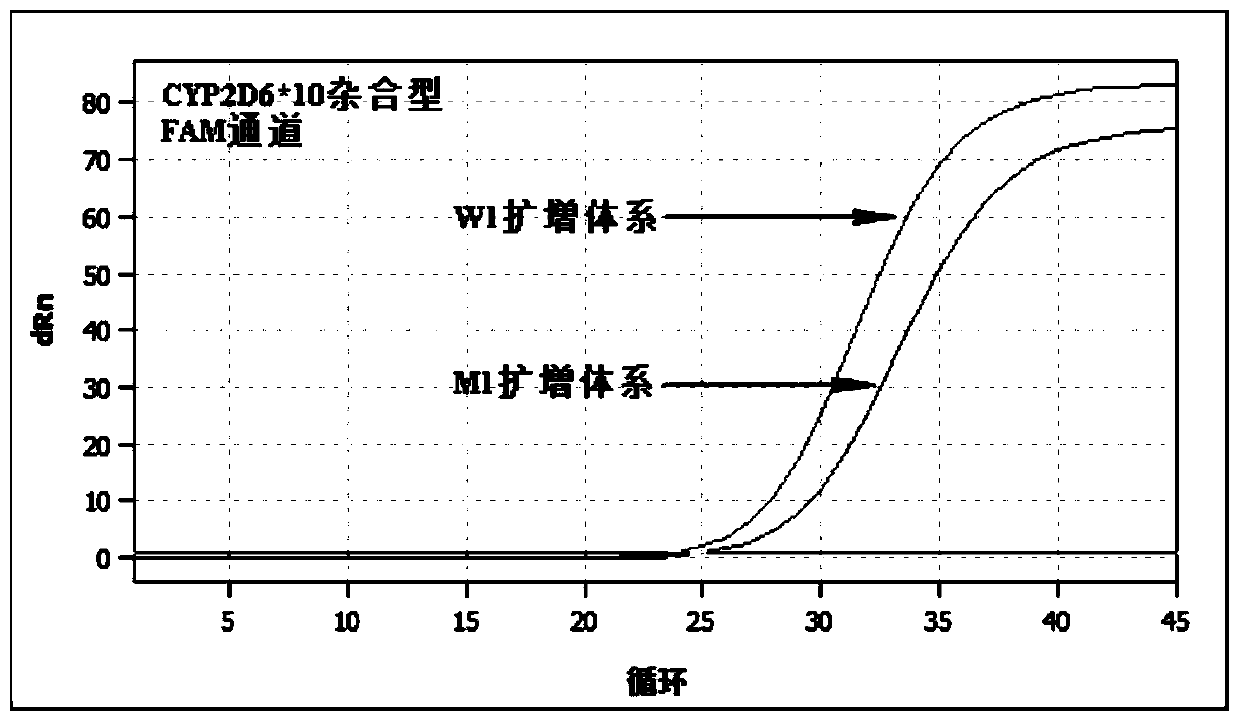
[0133] Table 4 wild type reaction system W1
[0134]
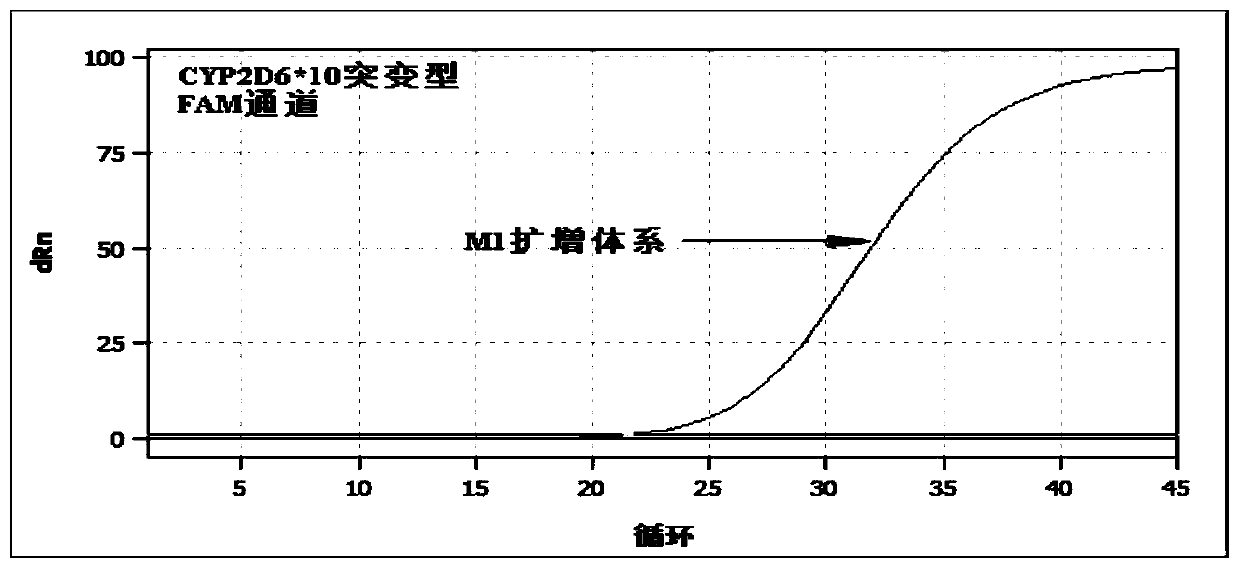
[0135] Table 5 mutant reaction system M1
[0136]
[0137]
[0138] Table 6 Wild type reaction system W2
[0139]
[0140] Table 7 Mutant reaction system M2
[0141] PCR reaction solution (M2) PCR reaction solution (E) sample DNA positive control negative control 20 μL 10μL 50μL - - 20 μL 10μL - 50μL - 20 μL 10μL - - 50μL
[0142] ② On-board inspection
[0143] Directly detect on the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com