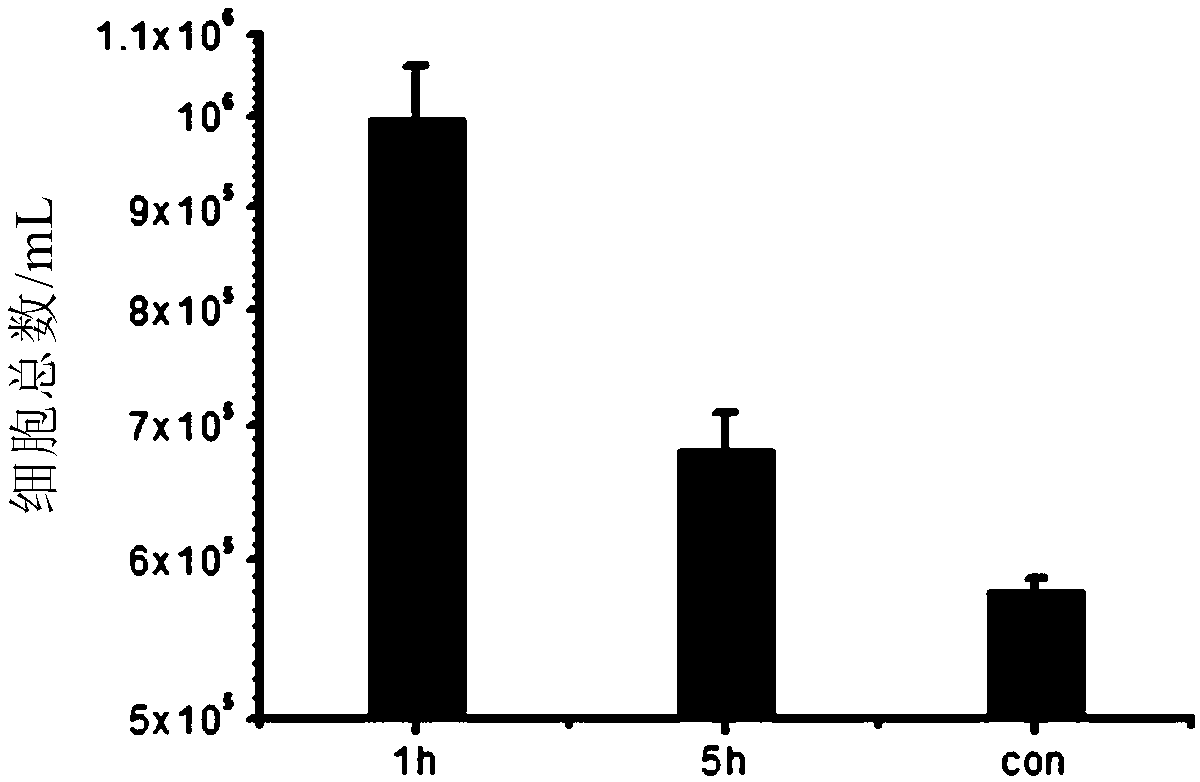
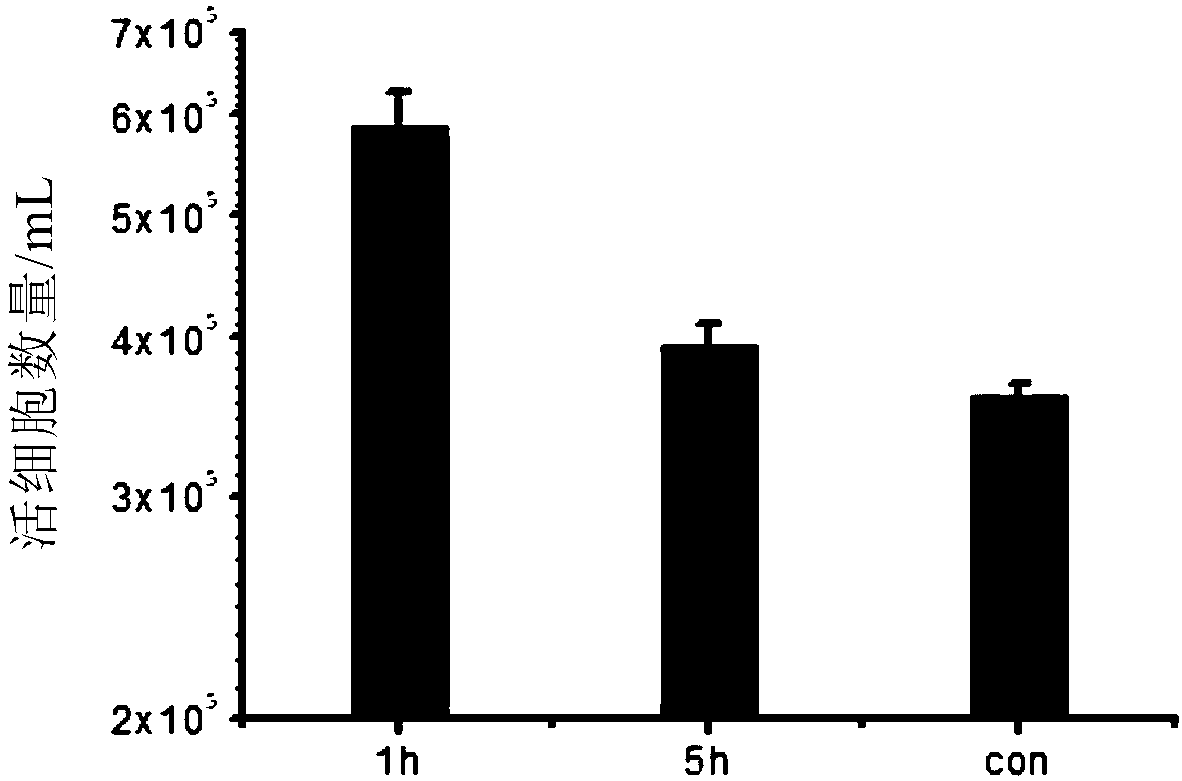
Method for preparing cells over-expressing exogenous genes
A technology of exogenous genes and cells, applied in the field of preparing cells overexpressing exogenous genes, can solve the problems of low transfection efficiency, high death rate of immune effector cells, excessive cell death, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Isolation and culture of liver cancer tissue-derived TIL cells
[0058] Collect freshly resected HCC specimens and process them immediately under sterile conditions. The specific method is as follows: remove the normal tissue and necrotic area around the liver cancer specimen, and remove the 1-2mm in size from different areas of the specimen 3 Place a small tissue block in each well of a 24-well plate. Add 2 mL of complete medium (GT-T551 medium containing 10% FBS) and 3000 IU / mL IL-2 to each well. Place the 24-well plate at 37 °C, 5% CO 2 cultured in an incubator. On the 5th to 6th day after the initiation of culture, a half-volume medium change was performed for all wells. Afterwards, according to the growth of TILs, a half-volume medium change was performed every 1-2 days. Once the wells were overgrown with TILs and all adherent cells had been removed, the TILs in each overgrown well were collected.
Embodiment 2
[0059] Embodiment 2: Construction of recombinant expression vector
[0060] The coding sequence of EGFP was artificially synthesized and loaded between the EcoRI and SalI restriction sites of vectors pNB328 and pS328 based on the PB transposon system (see CN201510638974.7 for the structure and sequence of pNB328, the entire contents of which are cited herein Incorporated in this article; compared with pNB328, pS328 lacks the PB transposon sequence; the EGFP coding sequence is shown in SEQ ID NO:9 in CN201510638974.7), and the constructed recombinant expression vectors were named pNB328-EGFP and pS328-EGFP respectively .
[0061] The coding sequence of PB transposase is artificially synthesized, and it is loaded between the EcoRI and SalI restriction sites of pS328 based on the PB transposon system. The coding sequence of PB transposase is as SEQ ID NO: 5 in CN201510638974.7 As shown, the constructed recombinant expression vector was named pS328-PB.
Embodiment 3
[0062] Example 3: Preparation of TIL expressing EGFP by electroporation of pNB328-EGFP (TIL-single transfection plasmid)
[0063] Follow the steps below to electroporate TIL-single transfection expressing EGFP:
[0064] 1) Add AIM-V medium to 3 wells in a 12-well plate in advance, numbered a, b, c, 2 mL per well, and then transfer to a cell culture incubator at 37°C 5% CO 2 Preheat for 1 hour;
[0065] 2) According to the table below, the ratio of the electrotransfer solution for a single dosage per well:
[0066] 100 μL Nucleocuvette TM Strip (μL)
Nucleofector TM volume of solution
82 Electroporation Supplementary Solution 18
[0067] 3) Get the TIL obtained in Example 1 into 3 EP tubes, and add 5×10 6 centrifuge at 1200rpm for 5min, discard the supernatant, then resuspend the cells with 500μL normal saline, and repeat the centrifugation step to wash the cell pellet;
[0068] 4) Add 6 μg of plasmid pNB328-EGFP to the electrotransfer sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com