Alda-1 solution as well as preparation method and application thereof
A solution and solvent technology, used in the field of medicine, can solve the problems of low solubility of Alda-1 and the inability of the dissolution method to be used in clinical applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 Alda-1 injection
[0052] Solvents: ethanol, polyethylene glycol 400, normal saline
[0053] Accurately weigh 20mg of Alda-1, add it to a clean 5ml ep tube, add ethanol to the ep tube, and use ultrasonic vibration for 3 minutes; add peg400 and normal saline, mix well, and sterilize to get ready. Wherein, the consumption situation of solvent is shown in Table 1.
[0054] Table 1 Solvent consumption situation
[0055] solvent ethanol polyethylene glycol 400 normal saline Preparation Example 1 1mL 600μL 400μL Preparation example 2 1mL 600μL 800μL Preparation example 3 1mL 1mL 400μL Preparation Example 4 1mL 1mL 800μL Preparation Example 5 1mL 1mL 600μL
[0056] The injections prepared in Preparation Examples 1-5 are all colorless transparent liquids, and their pH is in the range of 5-6.8. The injections prepared in Preparation Examples 1-5 above are stored at room...
experiment example 1
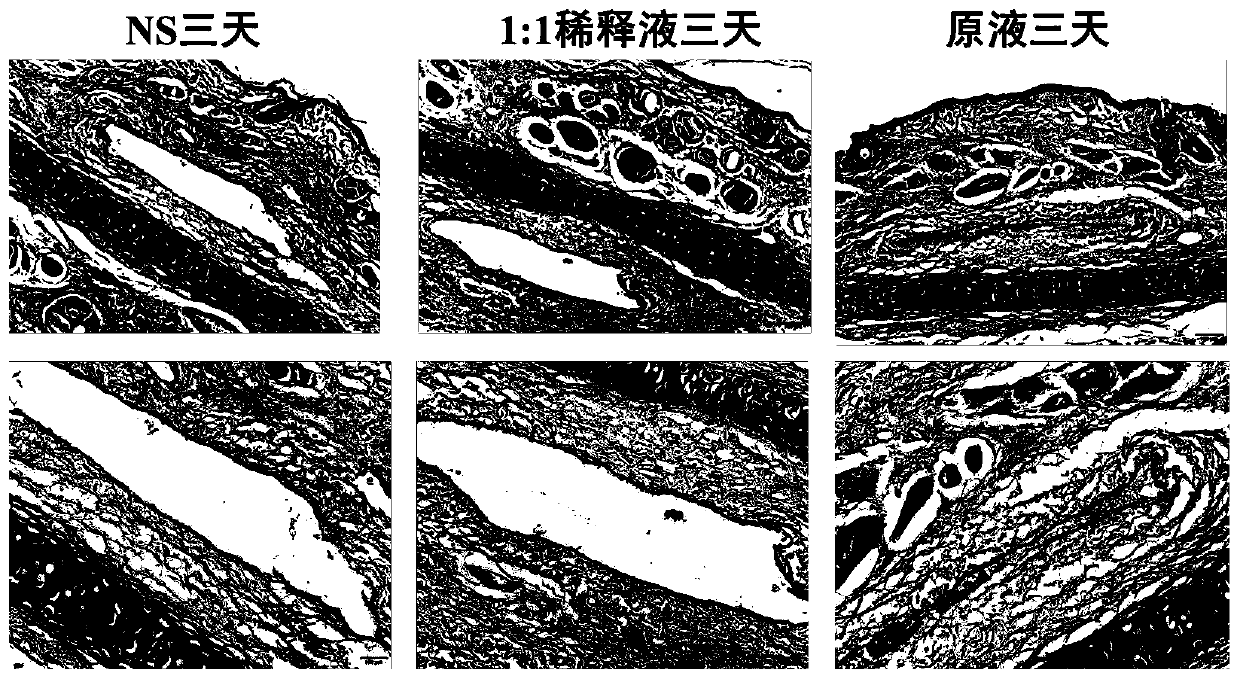
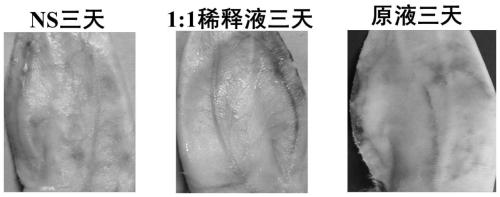
[0060] Experimental example 1, Alda-1 injection blood vessel stimulation test
[0061] Experimental method: Get 3 healthy rabbits, inject Alda-1 injection stock solution (taken from Preparation Example 1) (stock solution group), Alda-1 injection stock solution (preparation example 1 ) normal saline 1:1 dilution (1:1 dilution group), 0.9% sodium chloride injection (NS group), the injection volume is 1mL / kg, each adopts its own control, and the right ear margin is administered iv, etc. Volume 0.9% sodium chloride injection, 1 time / d, continuous administration for 3 days. 48 hours after the last administration, observe with the naked eye whether there is redness, swelling, hyperemia and other irritations at the injection site, and then kill the animal, and take three 1cm blood vessels and surrounding tissues continuously from the ear vein injection point to the centripetal direction, and add 4% neutral formaldehyde solution Fixed, routinely dehydrated, embedded in paraffin, cut ...
experiment example 2
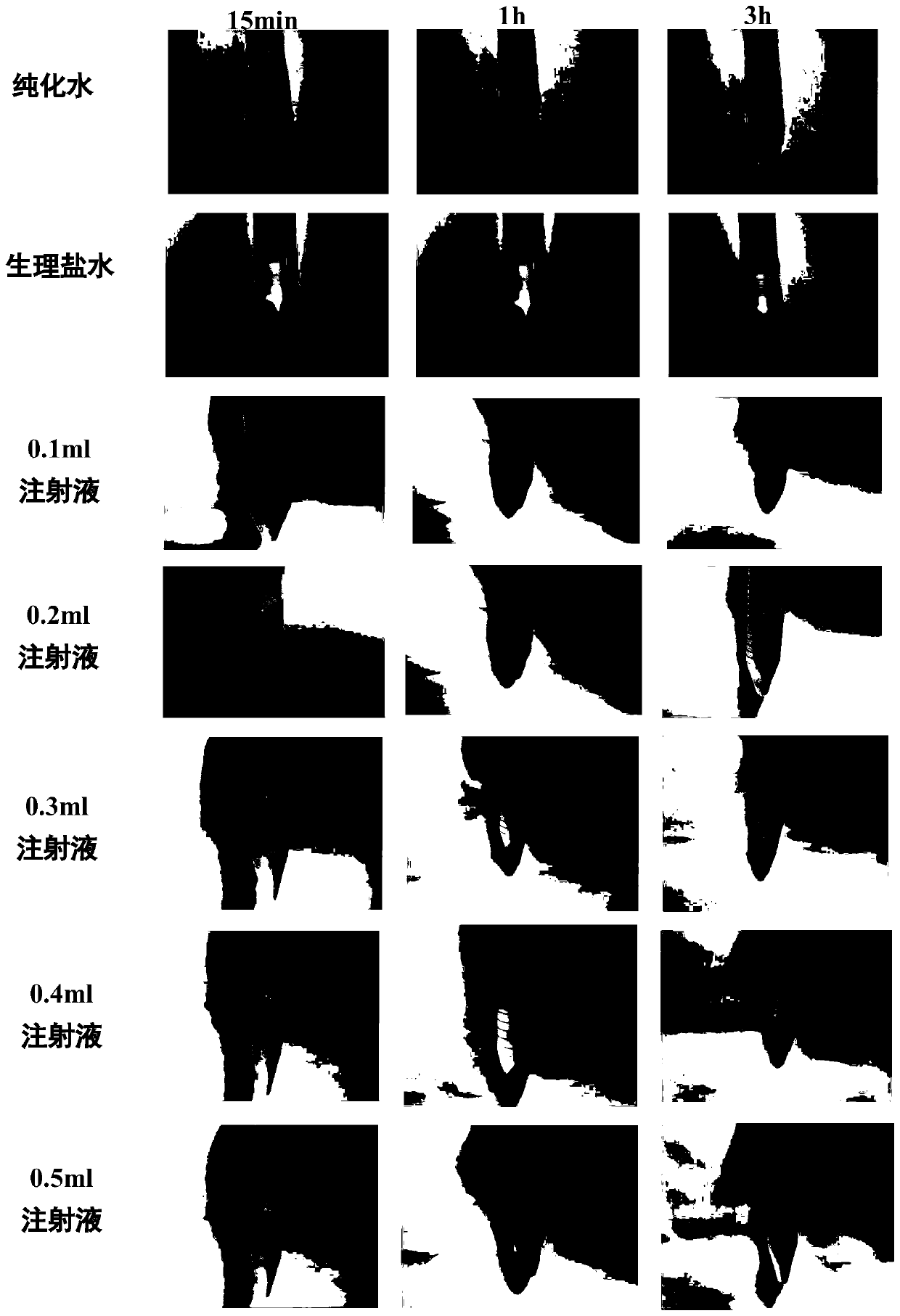
[0070] Experimental Example 2, Alda-1 Injection Hemolytic Test
[0071] Experimental method: take 1 healthy rabbit, take about 20mL of blood from the heart, place it in a triangular flask containing glass beads and shake it for 5 minutes to remove fibrin and make it into defibrinated blood. Transfer the defibrillated blood into a centrifuge tube, add normal saline and shake gently, then centrifuge at 1500r / min for 15min, discard the supernatant, add normal saline to mix and centrifuge, repeat 2-3 times, until the supernatant does not appear red, The obtained erythrocytes were diluted with physiological saline according to their volume to form a 2% suspension for later use. Take a clean centrifuge tube and add different volumes of Alda-1 injection stock solution (preparation example 1) in sequence, i.e. the injection group, 2 tubes for each concentration in parallel, and set a negative control (normal saline) and a positive control (purified water) at the same time. After all ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com