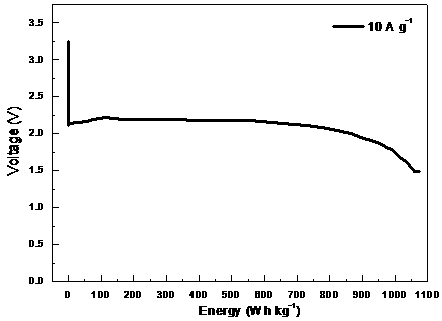
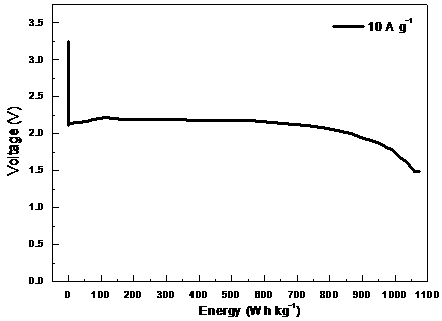
Preparation method and application of carbon fluoride material
A fluorinated carbon and fluorinated technology, applied in the field of electrochemical energy storage of power supply technology, to achieve the effect of high specific energy and high specific power
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A preparation method of carbon fluoride material, comprising the steps of:
[0024] Step 1: According to the mass ratio of the hard carbon raw material sucrose and the soft carbon raw material non-graphitized mesocarbon microspheres as 90%:10%, the non-graphitized mesocarbon microspheres and the non-graphitized mesocarbon microspheres are placed in the Mix in deionized water, add a little ethanol solution, stir evenly, and then sinter in a protective atmosphere at 2500 °C for 24 hours to obtain a pretreated material.
[0025] Step 2: Grinding the pretreated product obtained in step 1 until the particle size reaches below 60 microns, passing through 200 mesh, 400 mesh, and 800 mesh sieve successively to obtain a powdery product.
[0026] Step 3: Put the powder material obtained in Step 2 into the fluorination equipment, pass in fluorinated gas NF3, keep the pressure at 120kPa, and react at 400°C for 8 hours to obtain fluorine containing graphite carbon in the bulk phase ...
Embodiment 2
[0030] A preparation method of carbon fluoride material, comprising the steps of:
[0031] Step 1: According to the mass ratio of the hard carbon raw material sucrose and the soft carbon raw material non-graphitized mesocarbon microspheres as 70%:30%, the non-graphitized mesocarbon microspheres and the non-graphitized mesocarbon microspheres are placed in the Mix in deionized water, add a little ethanol solution, stir evenly, and then sinter in a protective atmosphere at 1500°C for 12 hours to obtain a pretreated material.
[0032] Step 2: Grinding the pretreated product obtained in step 1 until the particle size reaches below 60 microns, passing through 200 mesh, 400 mesh, and 800 mesh sieve successively to obtain a powdery product.
[0033] Step 3: Put the powder material obtained in Step 2 into the fluorination equipment, pass in fluorinated gas NF3, keep the pressure at 90kPa, and react at 350°C for 16 hours to obtain fluorine containing graphite carbon in the bulk phase a...
Embodiment 3
[0036] A preparation method of carbon fluoride material, comprising the steps of:
[0037] Step 1: According to the mass ratio of the hard carbon raw material sucrose and the soft carbon raw material non-graphitized mesocarbon microspheres as 98%: 2%, the non-graphitized mesocarbon microspheres and the non-graphitized mesocarbon microspheres are placed in the Mix in deionized water, add a little ethanol solution, stir evenly, and then sinter in a protective atmosphere at 2800°C for 22 hours to obtain a pretreated material.
[0038] Step 2: Grinding the pretreated product obtained in step 1 until the particle size reaches below 60 microns, passing through 200 mesh, 400 mesh, and 800 mesh sieve successively to obtain a powdery product.
[0039] Step 3: Put the powder material obtained in Step 2 into the fluorination equipment, pass in fluorinated gas NF3, keep the pressure at 100kPa, and react at 450°C for 16 hours to obtain fluorine containing graphite carbon in the bulk phase ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific power | aaaaa | aaaaa |
| Specific power | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com