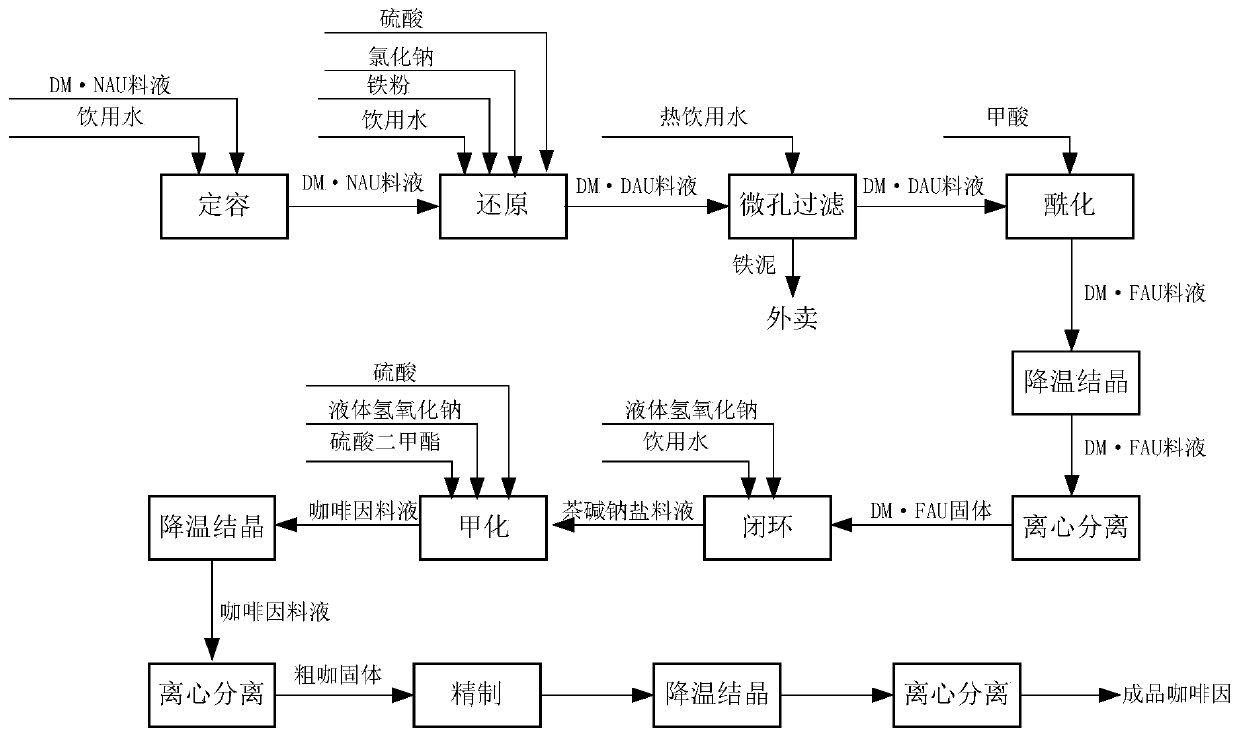
Post-treatment method for preparing 1,3-dimethyl-4-iminourazine
A technology of imidecarbazine and dimethylcyanoacetylurea, which is applied in the field of chemical technology, can solve the problems of increased environmental protection treatment costs, increased sulfuric acid consumption, poor control stability, etc., and achieves the goal of improving internal quality and reducing unit consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
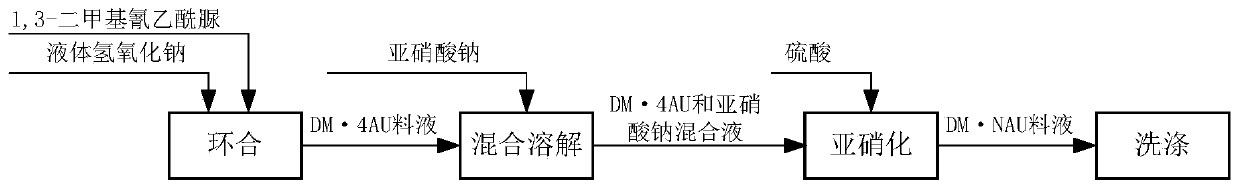
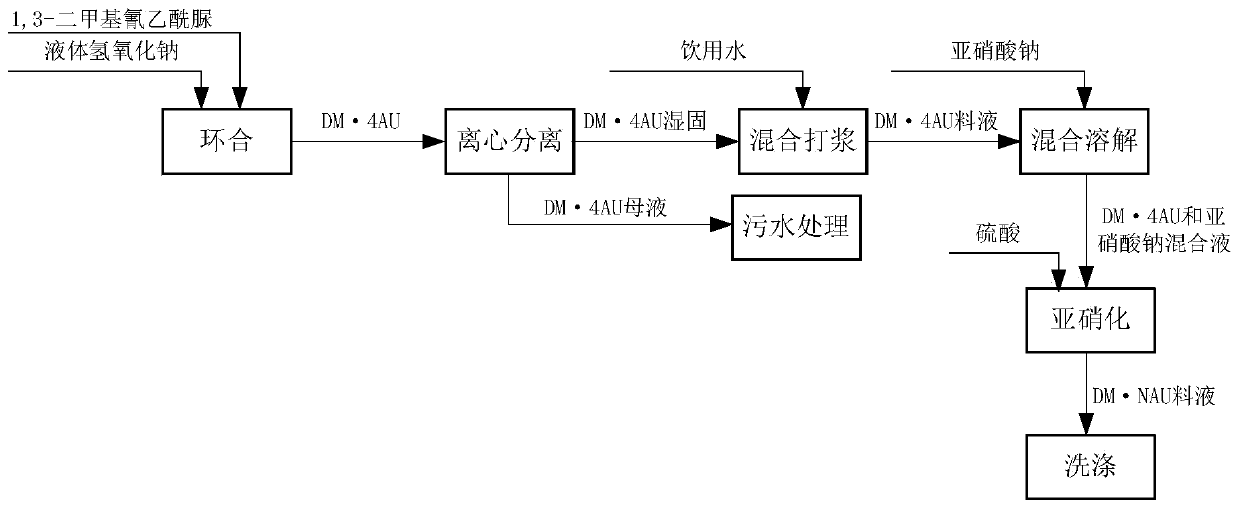
[0041] Transfer 1200L dimethylcyanoacetylurea into the cyclization reaction tank, adjust the base temperature of the cyclization reaction to 40°C, add 45L liquid sodium hydroxide to carry out the cyclization reaction to obtain DM·4AU, and the pH value at the end of the cyclization reaction is 9.2-9.4, The end point temperature is 90°C;
[0042] After the closed loop is completed, keep the temperature at 90°C, keep the temperature for 0.5 hours, and test the pH value during the heat preservation process to stabilize the pH value at 9.2 to 9.4 and keep it unchanged;
[0043]After the heat preservation is completed, cool down to 20°C for centrifugation, specifically: centrifuge 6 times, each centrifugation speed is 1500 rpm, and each centrifugation time is 20 minutes. After separation, 1,3-dimethyl-4- Urazine mother liquor and 460kg1,3-dimethyl-4-carbazine solid;
[0044] Sewage treatment of 1,3-dimethyl-4-imide ureazine mother liquor, 460kg of 1,3-dimethyl-4-imide ureazine soli...
Embodiment 2
[0046] Transfer 1200L dimethyl cyanoacetylurea into the cyclization reaction tank, adjust the base temperature of the cyclization reaction to 45°C, add 50L liquid sodium hydroxide to carry out the cyclization reaction to obtain DM·4AU, and the pH value at the end of the cyclization reaction is 9.2-9.4, The end point temperature is 95°C;
[0047] After the closed loop is completed, keep the temperature at 95°C for 0.5 hours and test the pH value during the heat preservation process to stabilize the pH value at 9.2 to 9.4 and keep it unchanged;
[0048] After the heat preservation is completed, cool down to 25°C for centrifugation, specifically: centrifuge 7 times, each time at a speed of 1500 rpm, and each time of centrifugation for 20 minutes, after separation, 1,3-dimethyl-4- Urazine mother liquor and 480kg1,3-dimethyl-4-carbazine solid;
[0049] Sewage treatment of 1,3-dimethyl-4-imide ureazine mother liquor, 480kg of 1,3-dimethyl-4-imide ureazine solid was put into the tra...
Embodiment 3
[0051] Transfer 1200L dimethyl cyanoacetylurea into the cyclization reaction tank, adjust the base temperature of the cyclization reaction to 42°C, add 54L liquid sodium hydroxide to carry out the cyclization reaction to obtain DM·4AU, and the pH value at the end of the cyclization reaction is 9.2-9.4, The end point temperature is 92°C;
[0052] After the closed loop is completed, keep the temperature at 92°C for 0.5 hours and test the pH value during the heat preservation process to stabilize the pH value at 9.2 to 9.4 and keep it unchanged;
[0053] After the heat preservation is completed, cool down to 22°C for centrifugation, specifically: centrifuge 6 times, each centrifugation speed is 1500 rpm, and each centrifugation time is 20 minutes. After separation, 1,3-dimethyl-4- Aminocarbazine mother liquor and 470kg1,3-dimethyl-4-imazinocarbazine solid;
[0054] Sewage treatment of 1,3-dimethyl-4-imide ureazine mother liquor, 470kg of 1,3-dimethyl-4-imide ureazine solid was p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com