Extraction-free SARS-CoV-2 nucleic acid detection method and kit
A coronavirus and kit technology, applied in the fields of biotechnology and medicine, can solve problems such as time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
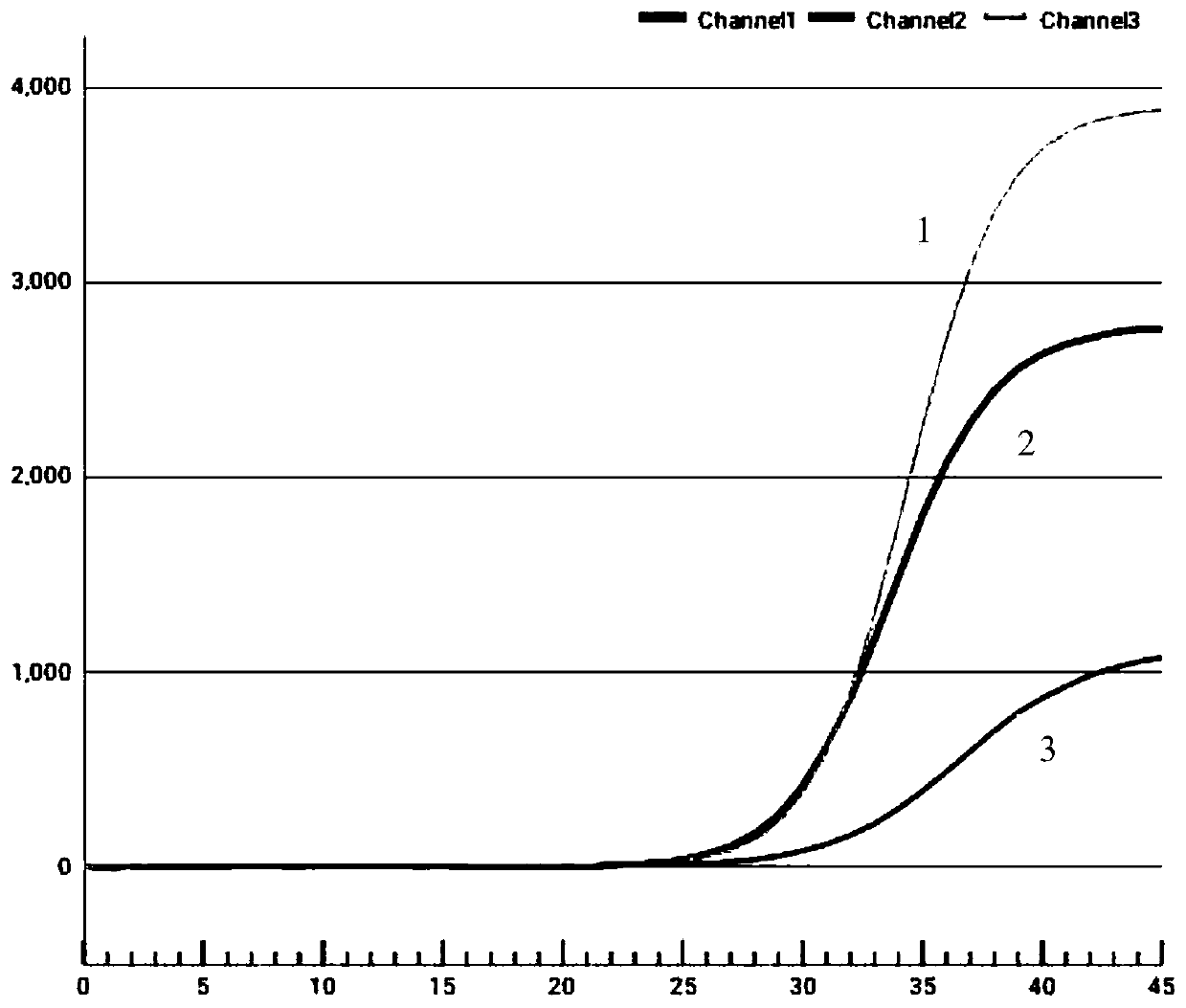
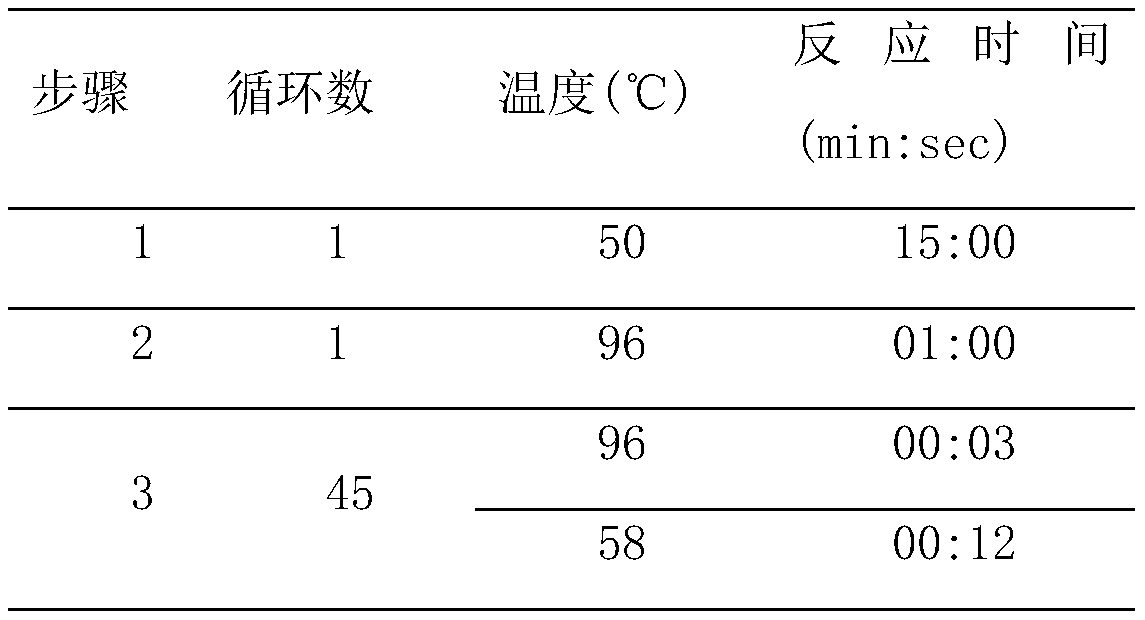
[0035] Example 1. Development of an extraction-free fluorescent PCR detection kit for novel coronavirus
[0036] 1. Design of primers and probes
[0037] Through the use of primer probe online design tools for the new coronavirus genome sequence published by the National Bioinformatics Center / National Genome Science Data Center http: / / www.yeastgenome.org / cgi-bin / web-primer Design specific primers and probes for ORF1ab gene and N gene. Considering specificity and amplification efficiency, the final selection of two sets of primer probe sequences is as follows:
[0038] The sequences of the ORF1ab gene-specific forward and reverse primers are 5'-GGCTTCACATATGTATTGTTC-3' and 5'-GCTCAAACTCTTCTTCTTCAC-3'; the sequence of the ORF1ab gene-specific probe is 5'-TCACCTTCTTCTTCATCCTCATCTGG-3', In addition, the two ends of the probe are respectively labeled with a fluorescence generating group FAM and a fluorescence quenching group BHQ1;
[0039] The sequences of the N gene-specific forward an...
Embodiment 2
[0051] Example 2. Detection of actual clinical samples
[0052] 1. Types of clinical samples The clinical samples used in this example come from throat swab samples collected by the Zhejiang Provincial Center for Disease Control and Prevention. The swabs collected are stored in normal saline or virus preservation solution. There are 301 cases, of which 105 are positive samples. 106 negative samples.
[0053] 2. The reference kit uses the kit used by the Zhejiang Provincial Center for Disease Control and Prevention as the reference reagent.
[0054] 3. Test results
[0055] 301 cases were selected for testing, of which 2 samples were thick sputum and the type of samples required by the kit were inconsistent and therefore were eliminated. 4 cases were excluded because the internal standard was not found and the test results were invalid. A total of 295 valid cases were excluded. All valid results are included in statistical analysis.
[0056]
[0057] 4. Result analysis
[0058] The resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com