Method for detecting milnacipran isomers
A detection method and technology of milnacipran, which is applied in the field of detection of milnacipran isomers, can solve the problem of not finding an efficient, simple and sensitive analytical method, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
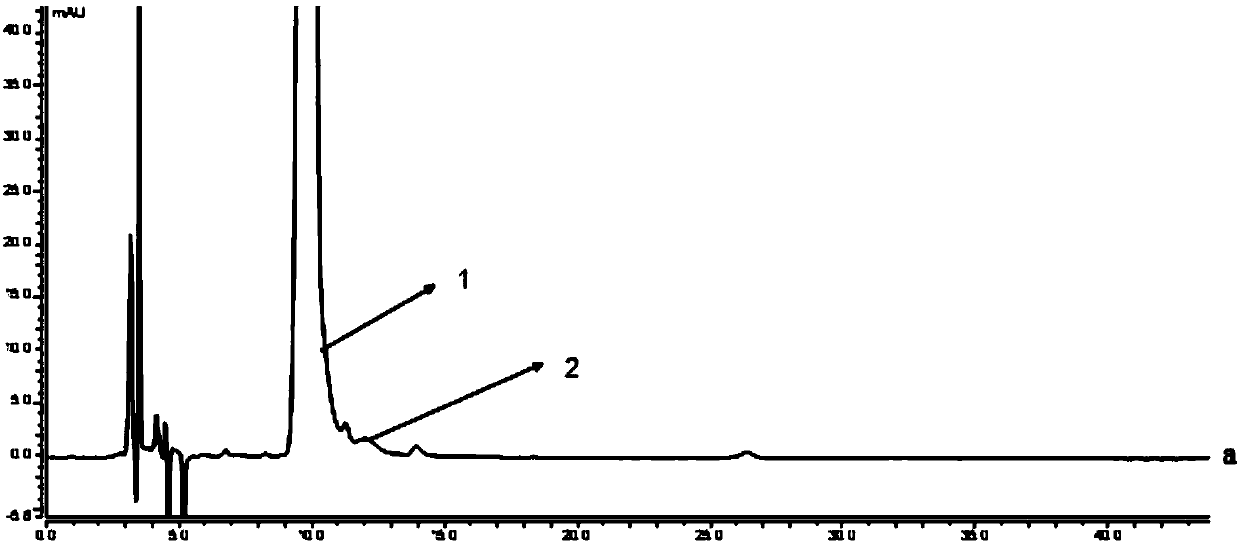
[0105] The preparation method of need testing solution is as follows: Accurately take by weighing appropriate amount of contents of self-made milnacipran hydrochloride sustained-release capsules (approximately equivalent to 5 mg of levomilnacipran) and put in a 10ml measuring bottle, add 1ml of ethanol, shake to disperse into independent Add a proper amount of diluent (n-hexane-ethanol=1000:25, v / v) to the pellet, ultrasonically for 30 minutes, let it cool, add diluent (n-hexane-ethanol=1000:25, v / v) to dilute to the mark, Shake well, filter, and take the continued filtrate as the test solution.
[0106] The preparation method of the system suitability solution is as follows: Accurately weigh an appropriate amount of the contents of the self-made milnacipran sustained-release capsules (approximately equivalent to 5 mg of levomilnacipran) and put it in a 10ml measuring bottle, add 1ml of ethanol to oscillate to disperse into independent pellets, Precisely add an appropriate amo...
Embodiment 2
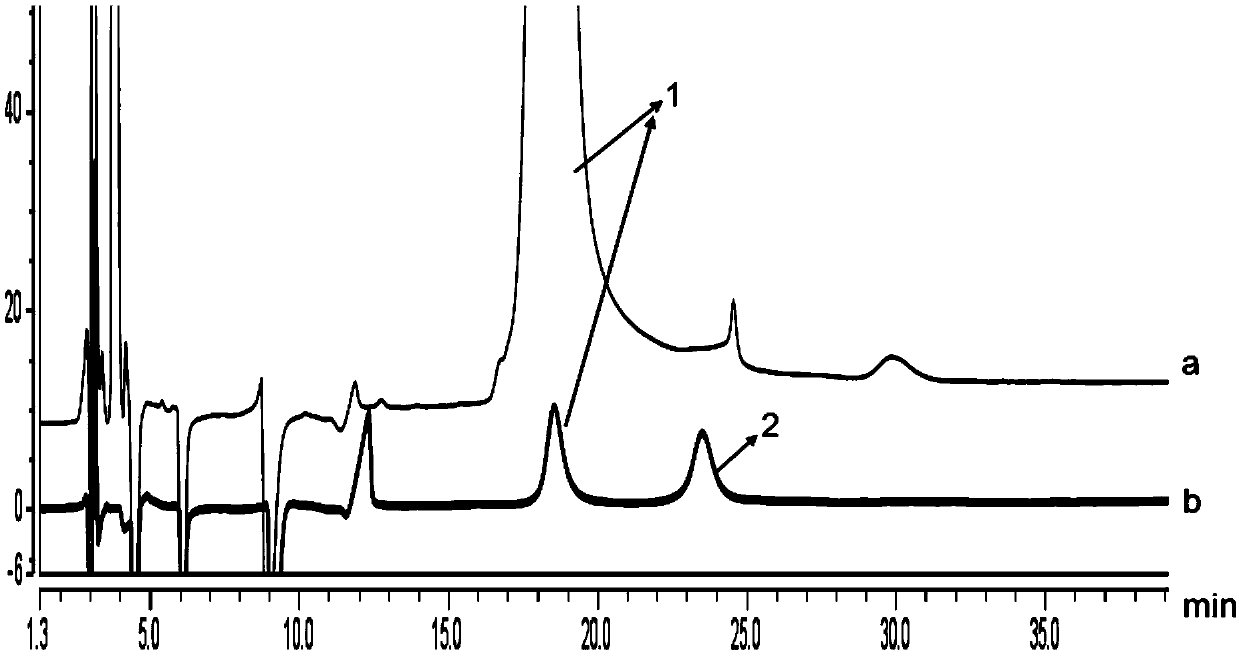
[0113] Test solution: Take a few commercially available milnacipran hydrochloride tablets, grind them finely, mix well, take an appropriate amount of fine powder (approximately equivalent to 25 mg of L-milnacipran) into a 50ml measuring bottle, add 5ml of ethanol to oscillate to disperse , add diluent (n-hexane-ethanol=1000:25, v / v) appropriate amount of ultrasonic treatment for 30min, add diluent (n-hexane-ethanol=1000:25, v / v) to dilute to the mark, shake well, filter, take Continue to obtain the filtrate.
[0114] The preparation method of the system suitability solution is as follows: Accurately weigh an appropriate amount of powder of commercially available milnacipran hydrochloride tablets (approximately equivalent to 5 mg of levomilnacipran) and put it in a 10 ml measuring bottle, add 1 ml of ethanol and oscillate to disperse into independent pellets, and precisely Add an appropriate amount of D-milnacipran reference substance, add an appropriate amount of diluent (n-he...
Embodiment 3
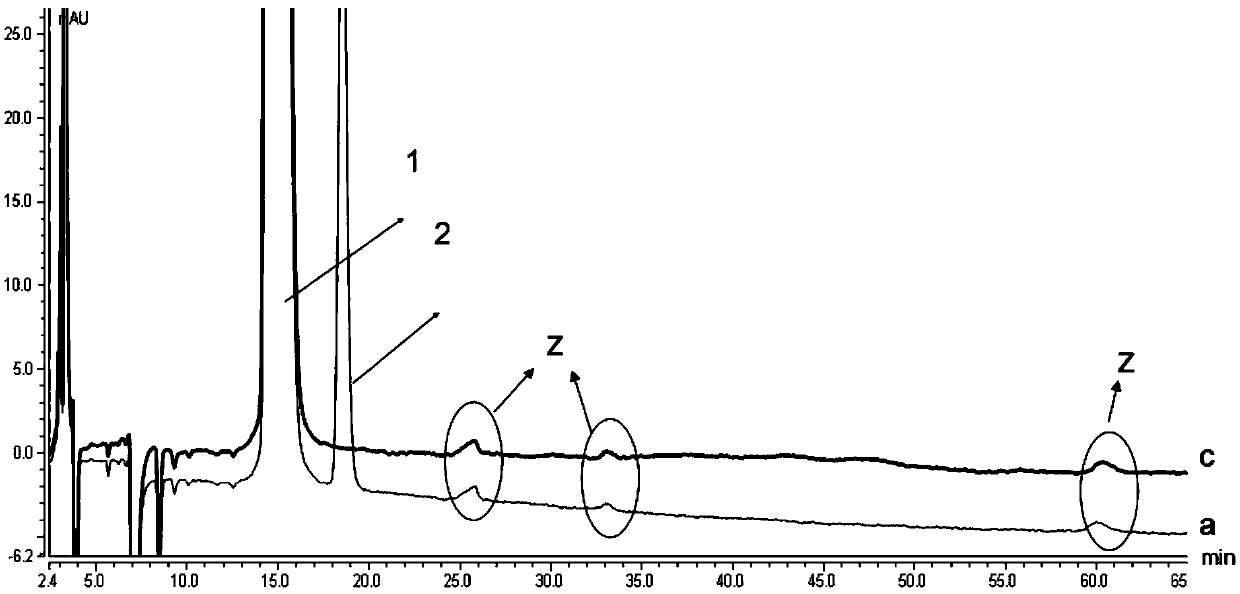
[0120] The preparation method of need testing solution is as follows: Accurately take by weighing commercially available milnacipran hydrochloride sustained-release capsule content (approximately equivalent to L-milnacipran 5mg) put 10ml measuring bottle, add ethanol 1ml, shake to disperse into independent Add a proper amount of diluent (n-hexane-ethanol=1000:25, v / v), ultrasonic for 30 minutes, let cool, add diluent (n-hexane-ethanol=1000:25, v / v) to dilute to the mark , Shake well, filter, and take the continued filtrate as the test solution.
[0121] The preparation method of the system suitability solution is as follows: Accurately weigh an appropriate amount of the content of the commercially available milnacipran hydrochloride sustained-release capsules (approximately equivalent to 5 mg of levomilnacipran) and put it in a 10 ml measuring bottle, add 1 ml of ethanol and oscillate to disperse into independent pellets , precisely add an appropriate amount of D-milnacipran r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com