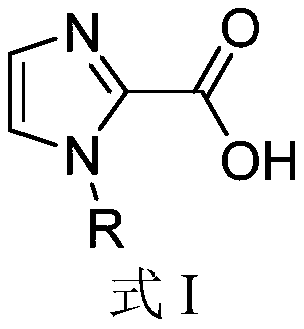
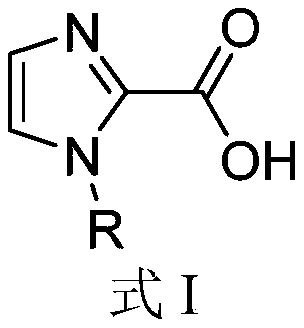
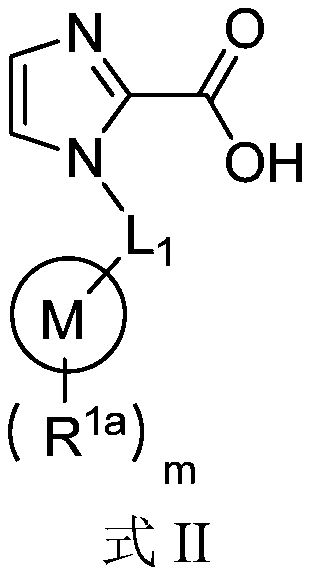
Application of 1-substituted-1H-imidazole-2-carboxylic acid compounds in preparation of metal beta-lactamase inhibitors
A compound and application technology, applied in the field of 1-substituted-1H-imidazole-2-carboxylic acid compounds, metallo-β-lactamase inhibitors, can solve the problem of no MBL inhibitors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Synthesis of 1-methyl-1H-imidazole-2-carboxylic acid (compound B):
[0071]
[0072] Dissolve imidazole-2-carboxylic acid ethyl ester 1 (100mg, 0.71mmol) in 10ml THF, slowly add sodium hydride (43mg, 1.78mmol) under ice bath to activate for 1h, then add methyl iodide (507mg, 3.57mmol), the addition is complete, React at room temperature for 1h. The reaction was completed by TLC detection, the reaction was stopped, the solvent was removed by concentration under reduced pressure, dissolved in water, extracted with ethyl acetate (3×20ml), the organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain a crude product. Purified by column chromatography (PE:EA=1:1) to obtain a yellow oily liquid, namely compound 1-methyl-1H-imidazole-2-carboxylic acid ethyl ester 1b (98mg, yield 89%).
[0073] The compound 1-methyl-1H-imidazole-2-carboxylic acid ethyl ester 1b (98mg, 0.63mmol) was dissolved in 6ml...
Embodiment 2
[0074] Example 2 Synthesis of 1-amino-1H-imidazole-2-carboxylic acid (compound 3):
[0075]
[0076] Dissolve imidazole 2-carboxylic acid (95mg, 0.86mmol) in 10ml N,N-dimethylformamide, add potassium tert-butoxide (154mg, 1.37mmol) under stirring, add O-(4-nitrogen) after activation for 0.5h Benzoyl) hydroxylamine (172 mg, 0.94 mmol). The reaction was performed at room temperature for 10 hours. TLC detected that the reaction was complete. The reaction was stopped. 3N HCl adjusted the Ph to about 5, solids separated out, filtered and washed with dichloromethane to obtain light yellow crystals, namely compound 1-amino-1H-imidazole-2-carboxylic acid ( Namely, compound 3, 82mg, yield 76.6%, HPLC purity>95%). 1 H NMR(400MHz, DMSO-d 6 )δ 7.53 (s, 1H), 7.08 (s, 1H), 6.79 (s, 1H), 6.17 (s br, 2H) ppm. 13 C NMR(101MHz, DMSO-d 6 )δ137.30,126.35,121.75ppm.ESI-MSm / z:128.04[M+H] + .
Embodiment 3
[0077] Example 3 Synthesis of 1-ethyl-1H-imidazole-2-carboxylic acid (compound 4):
[0078]
[0079] Ethyl imidazole-2-carboxylate 1 (100mg, 0.711mmol) was dissolved in 3ml of N,N-dimethylformamide, potassium carbonate (295mg, 21.4mmol) was added for activation for 30 minutes, and ethyl bromide (155mg, 1.41mmol). After the addition, the temperature was raised to 60°C and reacted for 4h. TLC detected that the reaction was complete. The reaction was stopped. Cooled to room temperature, diluted with water, extracted with ethyl acetate (3×20ml), combined the organic layers, washed with saturated brine, and anhydrous Dry over sodium sulfate, filter and concentrate to obtain crude product. Purified by column chromatography (PE:EA=2:1) to obtain a pale yellow liquid, namely compound 1-ethyl-1H-imidazole-2-carboxylic acid ethyl ester 3b (100 mg, yield 83.3%).
[0080] The compound 1-ethyl-1H-imidazole-2-carboxylic acid ethyl ester 3b (100mg, 0.59mmol) was dissolved in 6ml EtOH:H 2 To th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com