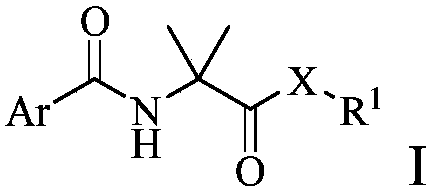
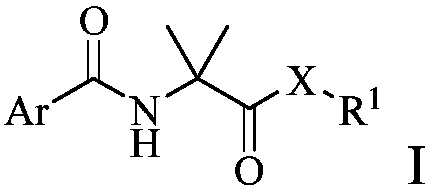
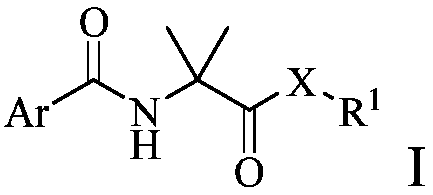
Arylamino isobutyryl derivative and application thereof
An aroylaminoisobutyryl derivative and alkylaminocarbonyl technology, which is applied in the field of chemistry and can solve the problems of few types of plant-induced disease resistance activators, affecting drug market promotion and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0161] Embodiment 1: the synthesis of compound 1-49
[0162]
[0163] In a 250mL flask, add 7.0 grams of 4-fluoro-2-chlorobenzoic acid, 10.0 grams of EDCI (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride), aminoisobutyric acid 7.5 grams of methyl ester hydrochloride, 150 mL of dichloromethane, and 5 mL of triethylamine. The reaction was stirred at room temperature for 12 hours. After the reaction was completed, the reaction liquid was washed with 50 mL×3 of water, the organic phase was dried with anhydrous sodium sulfate for 12 hours, precipitated under reduced pressure, 150 mL of ethanol and 7.0 g of sodium hydroxide were added, and the reaction was stirred at room temperature for 2 hours. After the completion of the reaction as monitored by TLC, desolvation under reduced pressure, add 100 mL of water to the residue, extract with ethyl acetate 50 mL × 3, dry the organic phase with anhydrous sodium sulfate for 12 hours, desolvate under reduced pressure, column c...
Embodiment 2
[0164] Embodiment 2: the synthesis of compound 4-7
[0165]
[0166] In a 250mL flask, add 3.1 grams of compound 4-fluorobenzamidoisobutyric acid (synthesized with reference to Example 1), EDCI (1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride Salt) 3.0 g, dichloromethane 150 mL. The reaction was stirred at room temperature for 12 hours. After the reaction was completed, the reaction solution was washed with 100 mL of water × 3, the organic phase was dried with anhydrous sodium sulfate for 12 hours, precipitated under reduced pressure, and the residue was column chromatographed (eluent was ethyl acetate and petroleum ether (boiling range 60-90 °C), the volume ratio is 1:4) to obtain the compound 2-(4-fluorophenyl)-4,4-dimethyl-2-oxazolin-5-one, 1.6 g of white solid.
[0167]
[0168] Into a 250 mL flask were added 1.0 g of the compound 2-(4-fluorophenyl)-4,4-dimethyl-2-oxazolin-5-one, 1.5 g of ethylamine, and 150 mL of tetrahydrofuran. The reaction was stirr...
Embodiment 3
[0169] Embodiment 3: the synthesis of compound 17-7
[0170]
[0171] Into a 250 mL flask were added 2.0 g of the compound 4-fluorobenzamidoisobutyric acid (synthesized with reference to Example 1), 4.0 g of oxalyl chloride, and 150 mL of dichloromethane. The reaction was stirred at room temperature for 12 hours. After the reaction was completed, the reaction solution was washed with 100 mL of water × 3, the organic phase was dried with anhydrous sodium sulfate for 12 hours, precipitated under reduced pressure, and the residue was column chromatographed (eluent was ethyl acetate and petroleum ether (boiling range 60-90 °C), the volume ratio is 1:4) to obtain the compound 2-(4-fluorophenyl)-4,4-dimethyl-2-oxazolin-5-one, 1.1 g of white solid.
[0172]
[0173] Into a 250 mL flask were added 1.0 g of the compound 2-(4-fluorophenyl)-4,4-dimethyl-2-oxazolin-5-one, 1.5 g of cyclohexylamine, and 150 mL of tetrahydrofuran. The reaction was stirred at room temperature for 12 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com