Bipolar organic photoelectric material based on N-C=O resonance structure, and preparation method and application thereof
A technology of organic optoelectronic materials and resonance structures, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of triplet energy level reduction, conjugated system extension, molecular band gap narrowing, etc., and achieve high triplet state Energy level, easy availability of raw materials, favorable effects on injection and transport
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation method of organic optoelectronic material 1
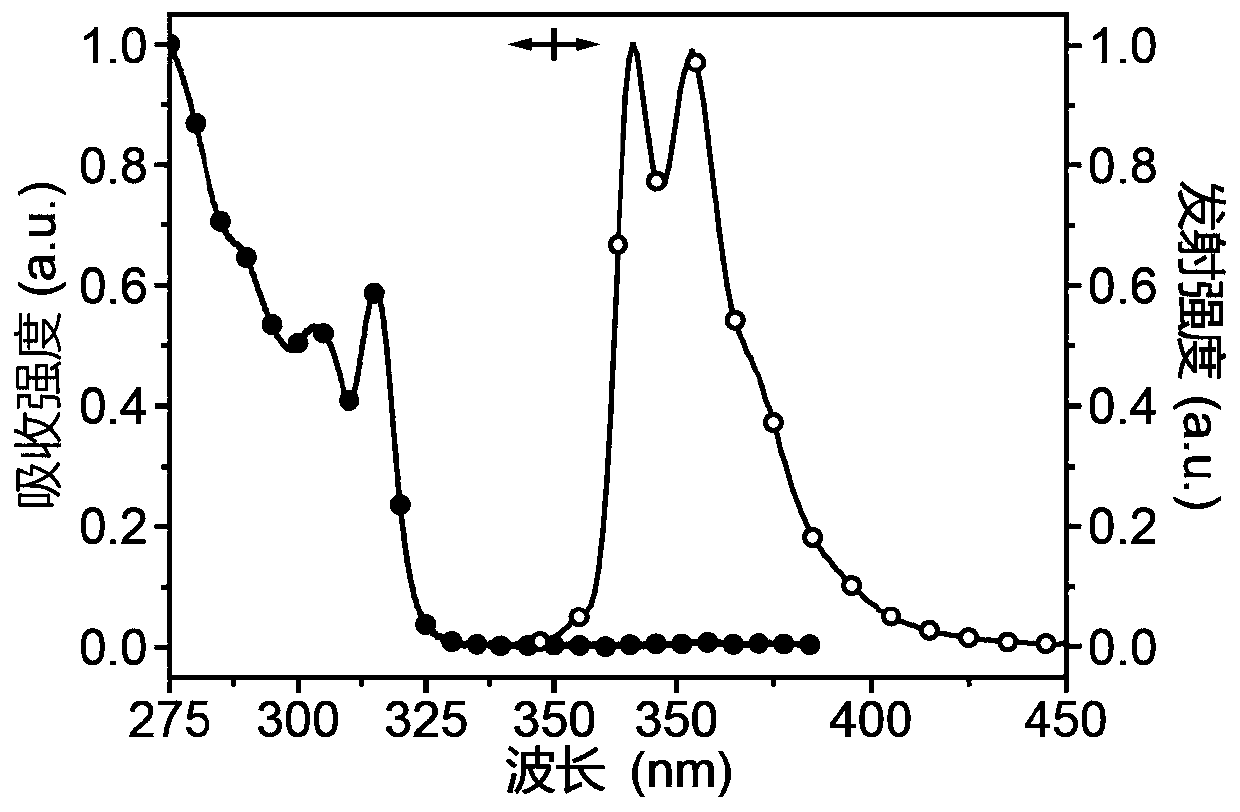
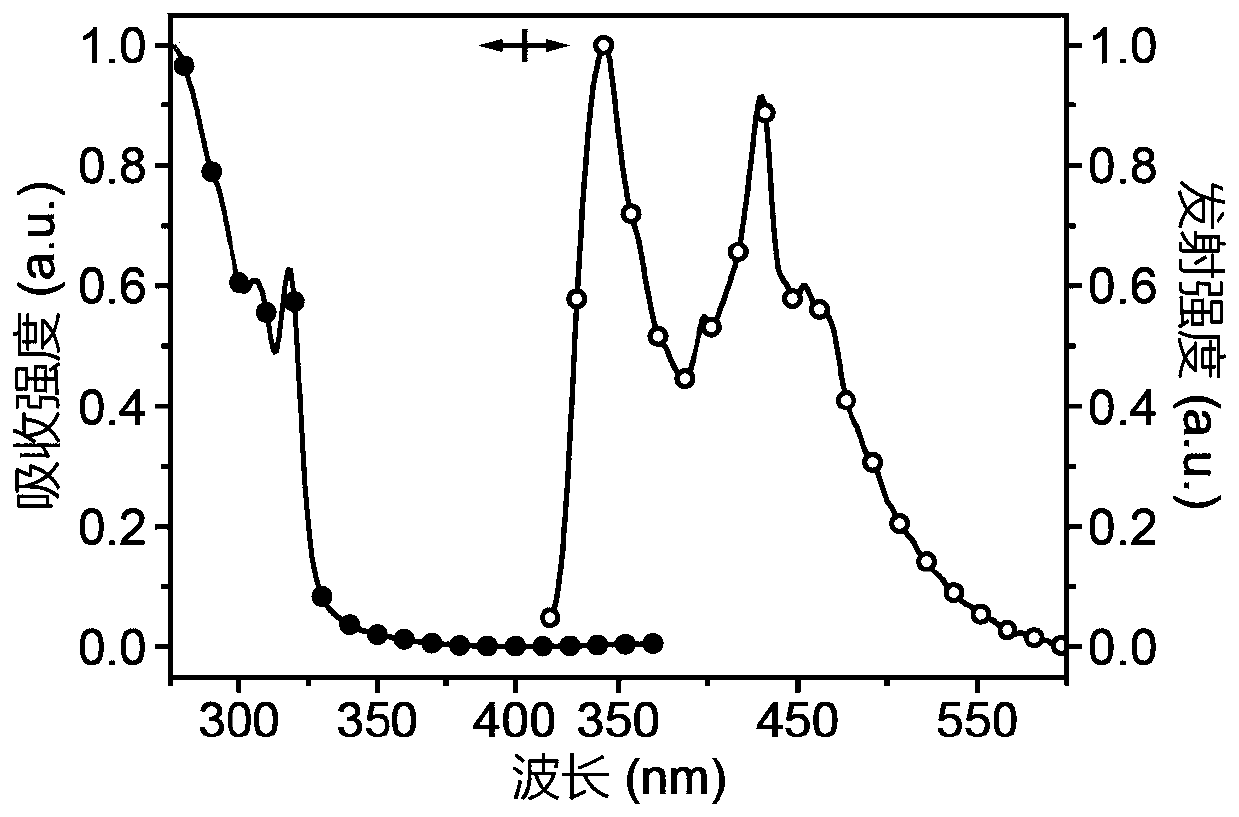
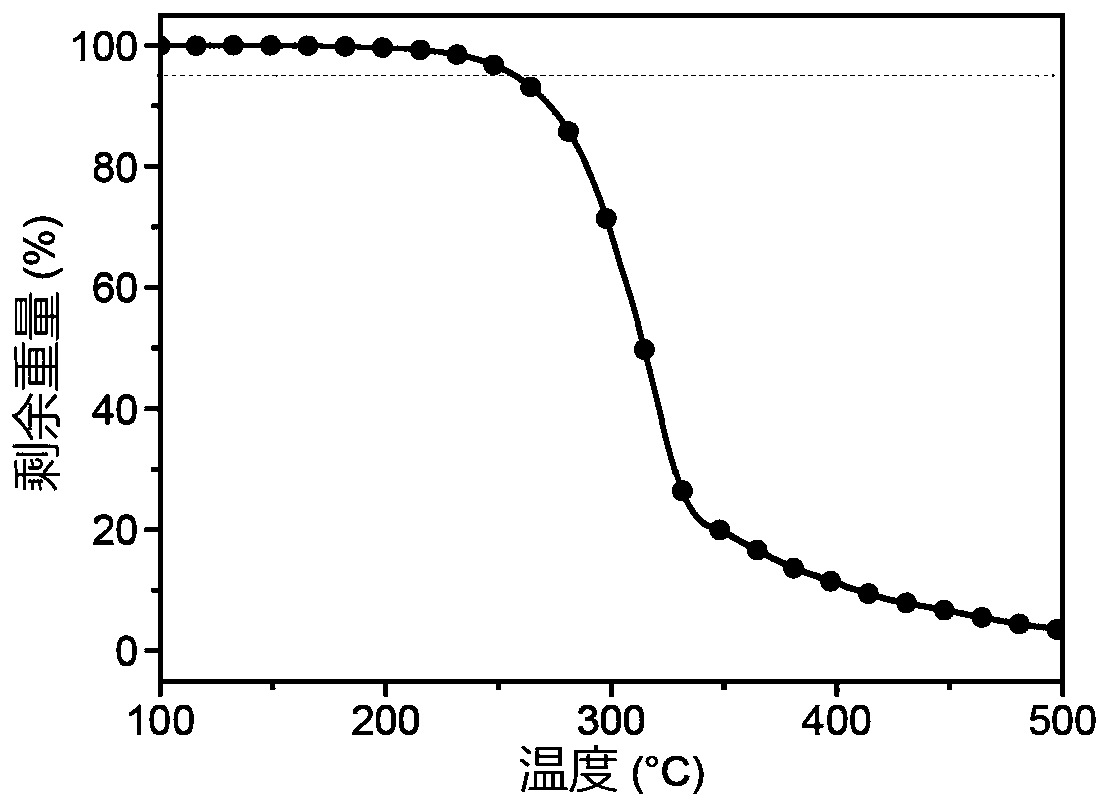
[0026] Take two reaction flasks, add 0.50g carbazole and 0.24g N,N-carbonyldiimidazole, vacuumize, blow argon three times, and under the protection of nitrogen, add 10mL ultra-dry dimethyl sulfoxide to dissolve the substrate. Heat and stir at 100-120°C for 6 hours. After the reaction, cool to room temperature, add deionized water to precipitate a white precipitate, filter with suction, collect the white solid and dissolve it in dichloromethane, dry it with anhydrous sodium sulfate, filter, and concentrate the filtrate , purified by column chromatography to give a white solid. Yield: 44%. 1 HNMR (DMSO-d 6 ,400MHz)δ(ppm):8.30(d,J=8Hz,4H),7.44-7.37(m,8H),7.43(d,J=8Hz,4H). 13 C NMR (CDCl 3 ,100MHz)δ(ppm):149.53,137.95,127.24,125.91,123.57120.20,114.58.HRMS(EI):m / zcalcdforC 25 h 16 N 2 O[M] + :361.1347; found: 361.1341..
[0027] The structure is as follows:
[0028]
Embodiment 2
[0029] Example 2: Performance characterization of phosphorescent organic electroluminescent devices (PhOLEDs)
[0030] In this embodiment, the device using the complex as the light-emitting layer may include: 1. a conductive glass layer (ITO); 2. a hole injection layer PEDOT:PSS; 3. a hole transport layer (TAPC); 4. an exciton blocking layer ( mCP); 5. Light emitting layer; 6. Electron transport layer (TmPyPB); 7. Electron injection layer (LiF); 8. Cathode Al.
[0031] The fabrication method of PhOLEDs is as follows: first spin-coat PEDOT:PSS hole injection layer on the cleaned glass substrate (ITO), and then sequentially vapor-deposit. ITO / PEDOT:PSS(30nm) / 1,3,5-Triazo-2,4,6-triphosphorine-2,2,4,4,6,6-tetrachloride(TAPC)(20nm) / N,N′- dicarbazolyl-3,5-benzene(mCP)(8nm) / host:18wt%FIrpic(22nm) / 1,3,5-tri(m-pyrid-3-yl-phenyl)benzene(TmPyPB)(35nm) / LiF (1nm) / Al(100nm). The host material in the light-emitting layer is the material 1 prepared in the above examples. The current densi...
Embodiment 3
[0032] Example 3: Performance characterization of thermally activated delayed fluorescence organic electroluminescent devices (TADF OLEDs)
[0033] In this embodiment, the device using small organic molecules as the light-emitting layer may include: 1. Conductive glass layer (ITO); 2. Hole injection layer PEDOT:PSS; 3. Light-emitting layer; 4. Exciton blocking layer (TSPO1); 5. . Electron transport layer (TPBi); 6. Electron injection layer (Liq); 7. Cathode Al.
[0034] The fabrication method of TADFOLEDs is as follows: first spin-coat the PEDOT:PSS hole injection layer on the cleaned ITO, and then vapor-deposit sequentially. ITO / PEDOT:PSS(30nm) / TAPC(20nm) / mCP(8nm) / host:30wt%bis-[4-(9,9-dimethyl-9,10-dihydroacridine)-phenyl]-sulfone(DMAC-DPS )(20nm) / diphenylphosphineoxide-4-(triphenylsilyl)phenyl(TSPO1)(5nm) / 1,3,5-tris(N-phenylbenzmidazol-2-yl)benzene(TPBi)(35nm) / LiF(1nm) / Al (100nm). The host material in the light-emitting layer is the material 1 prepared in the above examp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com