Organic blue light micromolecule based on phenanthroimidazole and application of organic blue light micromolecule in preparation of non-doped organic electroluminescent device
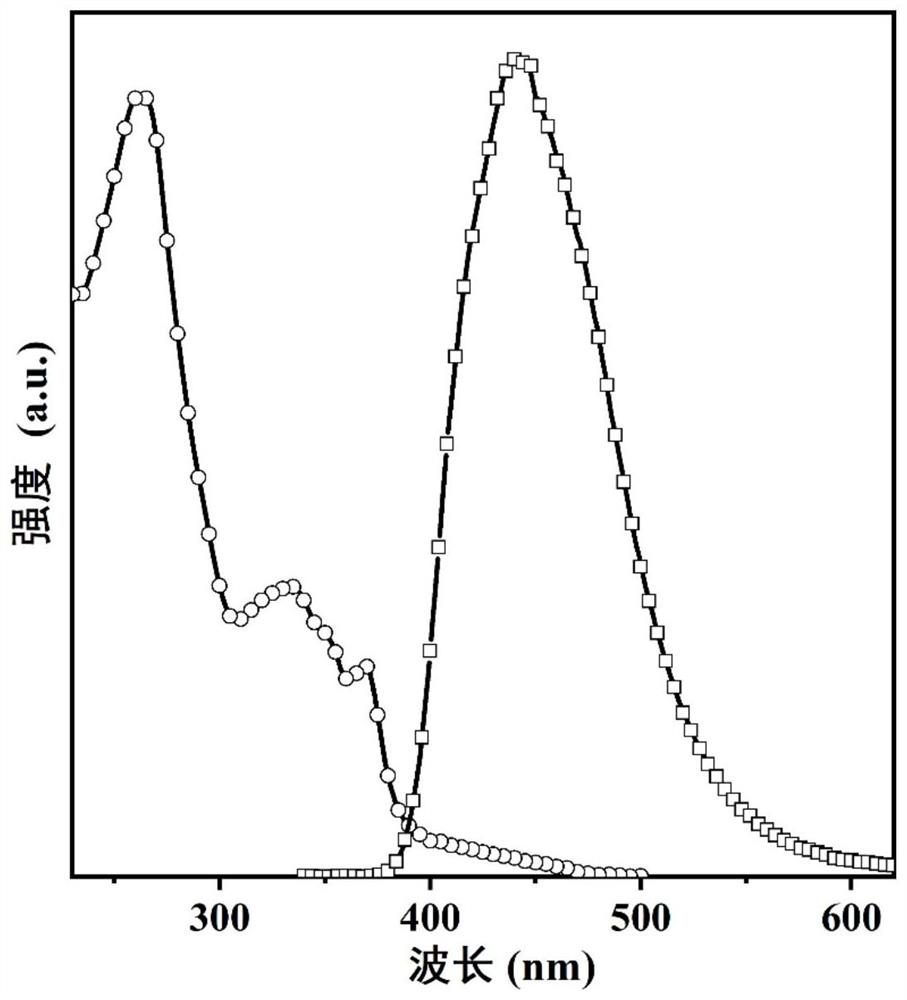
The technology of an electroluminescent device and phenanthroimidazole is applied in the application field of preparing non-doped organic electroluminescent devices, which can solve the problems of low color purity, roll-off, and low device efficiency, and achieve simple and high-efficiency preparation methods. Fluorescence quantum yield, the effect of realizing saturated blue light emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the preparation of compound P1 of this embodiment, the steps are as follows:
[0032]
[0033]Synthesis of M1: M1 was synthesized by one-pot method. In a 100 mL round-bottomed flask, phenanthrenequinone (2.08 g, 10.00 mmol), aniline (3.73 g, 40.00 mmol), 3,5-dibromobenzaldehyde (2.64 g, 10.00 mmol) and ammonium acetate (3.85 g, 50 mmol) were dissolved in 50 mL of glacial acetic acid solution, and heated to reflux at 120° C. for 2 hours. After the reaction, the reaction was quenched with water, and the solid was obtained by suction filtration, washed with water, glacial acetic acid and ethanol successively to obtain a crude product, which was separated and purified by column chromatography (petroleum ether: dichloromethane volume ratio=2:1) to obtain a white solid ( 4.23 g, yield: 80%). Mass MALDI-TOF (m / z) [M + ]: The measured value is 528.77, the theoretical value is 528.25.
[0034]
[0035] Synthesis of M2: M1 (5.28 g, 10.00 mmol), pinacol di...
Embodiment 2
[0038] Embodiment 2: the preparation of this embodiment compound P2, its steps are as follows:
[0039]
[0040] Synthesis of M3: M3 was synthesized by one-pot method. In a 100 mL round bottom flask, phenanthrenequinone (2.08 g, 10.00 mmol), aniline (3.73 g, 40.00 mmol), 4-bromobenzaldehyde (1.85 g, 10.00 mmol) and Ammonium acetate (3.85 g, 50 mmol) was dissolved in 50 mL of glacial acetic acid solution, and heated under reflux at 120° C. for 2 hours. After the reaction, the reaction was quenched with water, and the solid was obtained by suction filtration, washed with water, glacial acetic acid and ethanol successively to obtain a crude product, which was separated and purified by column chromatography (petroleum ether: dichloromethane volume ratio=2:1) to obtain a white solid ( 3.59 g, yield: 80%). Mass MALDI-TOF (m / z) [M + ]: The measured value is 449.55, the theoretical value is 449.35.
[0041]
[0042] Synthesis of M4: M3 (4.49 g, 10.00 mmol), pinacol diborona...
Embodiment 3
[0045] Embodiment 3: the preparation of this embodiment compound P3, its steps are as follows:
[0046]
[0047] The present embodiment is basically the same as embodiment 2, and its difference is: in this example, 5'-bromo-terphenyl needs to be replaced with 4-bromo-5'-phenyl-1,1' of equivalent amount: 3',1"-terphenyl as a white powdery solid (1.35 g, yield: 80%). Mass spectrum: MALDI-TOF (m / z) [M + ]: The measured value is 674.27, the theoretical value is 674.85; elemental analysis: C 51 H 34 N 2 Theorized C 90.77, H 5.08, N 4.15; Measured C 90.73, H 5.09, N 4.17.
PUM
| Property | Measurement | Unit |
|---|---|---|
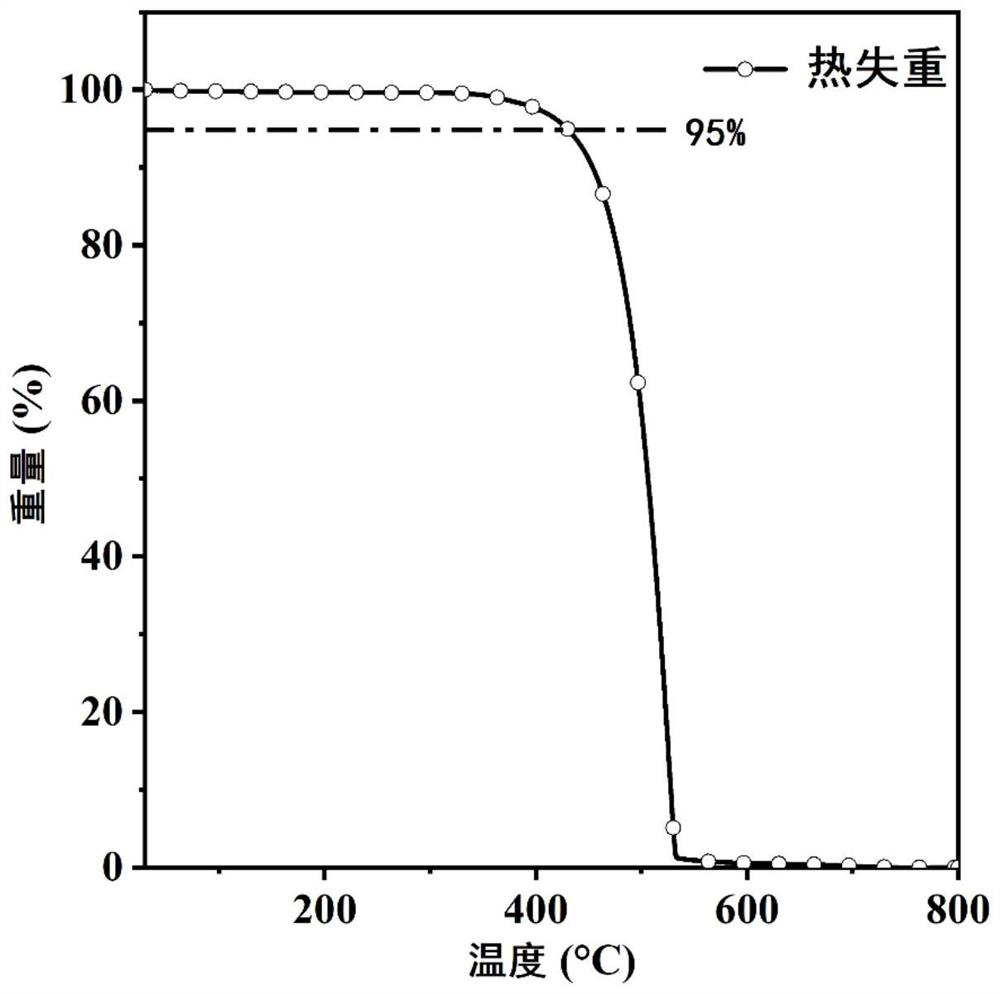
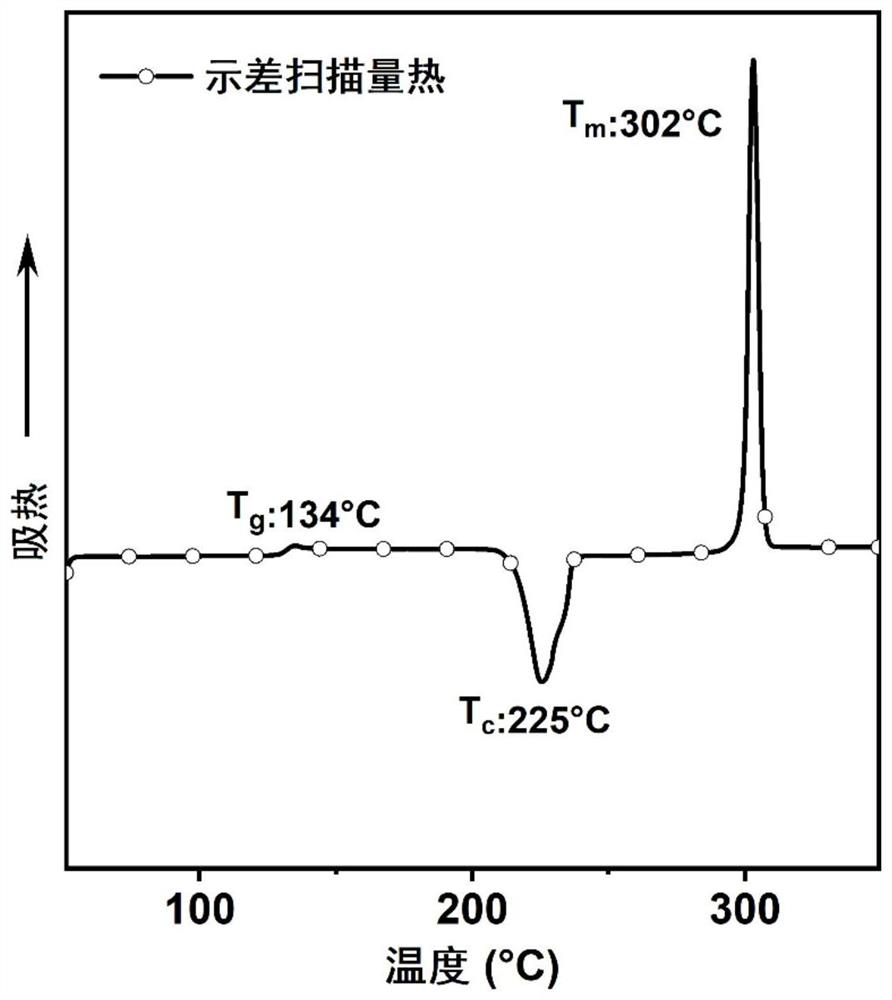
| glass transition temperature | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com