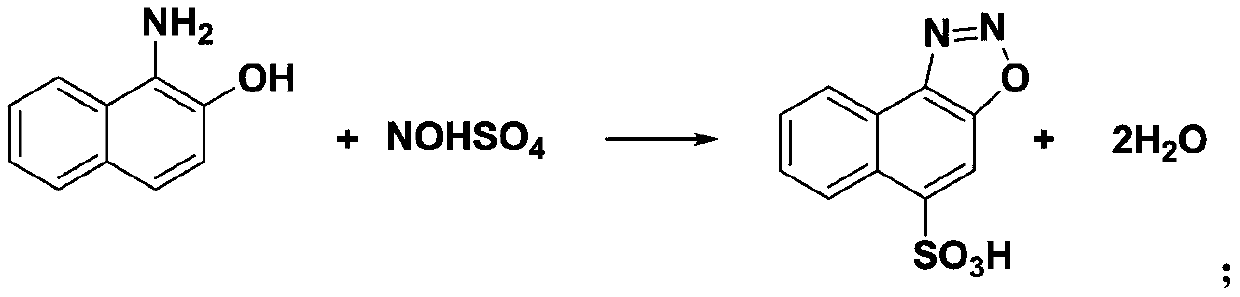
Method for preparing 6-nitro-2-diazo-1-naphthol-4-sulfonic acid hydrate
A technology of acid oxygen body and nitrate body, which is applied in the field of preparation of 6-nitro-1,2,4-acid oxygen body, can solve the problems of heating, consumption of co-solvent, increase of cost, etc., and achieve high reaction yield and reaction Yield improvement and cost reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
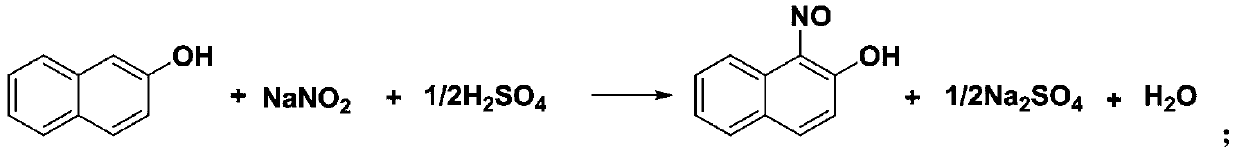
[0033] (1) Nitrosation reaction
[0034] Weigh 72.0 g of 2-naphthol and add it to 144.0 g of water, stir at room temperature for beating for 30 min, then add 84.5 g of 41% sodium nitrite solution, continue stirring for 10 min, and beat through pump A (the flow rate of the raw material solution is 11 mL / min). into the tubular reactor, and simultaneously pump 20% dilute sulfuric acid into the tubular reactor through pump B (the flow rate is 5mL / min). in, then filter at room temperature, and the filter cake is washed with water until the Congo red test paper does not change color, and 85.3 g of the yellow product is obtained, and the yield is 97%, and its content measured by HPLC is 98.6%.
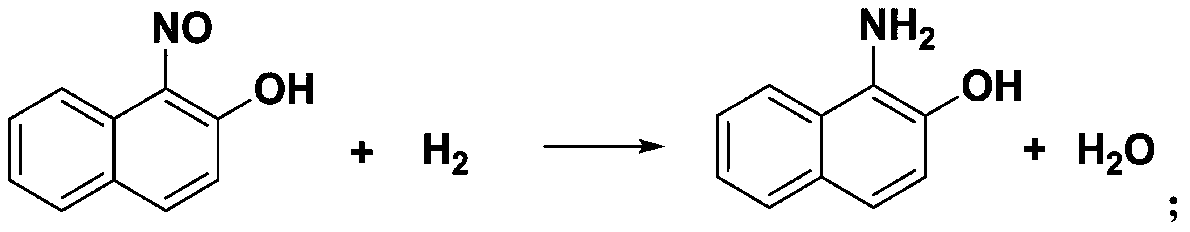
[0035] (2) Catalytic hydrogenation reaction
[0036] Add 83.4 g of the prepared 1-nitroso-2-naphthol into 417.0 g of water, stir at room temperature until dissolved, and simultaneously open pump C and hydrogen pressure reducing valve, pump C (flow rate is 2 mL / min), Hydrogen flows into the ...
Embodiment 2
[0042] (1) Nitrosation reaction
[0043] Weigh 72.0 g of 2-naphthol and add it to 360.0 g of water, stir at room temperature for beating for 30 min, then add 84.5 g of 41% sodium nitrite solution, continue stirring for 10 min, and beat through pump A (the flow rate of the raw material solution is 10 mL / min). into the tubular reactor, while pumping 50% dilute sulfuric acid into the tubular reactor through pump B (the flow rate is 2.1mL / min), the tubular reactor is placed at 30°C, the residence time is 5min, and the reaction solution flows into the bottle, then filter at room temperature, and the filter cake is washed with water until the Congo red test paper does not change color, and 82.5 g of the yellow product is obtained, and the yield is 93%, and its content measured by HPLC is 97.3%.
[0044] (2) Catalytic hydrogenation reaction
[0045]Add 82.5 g of the prepared 1-nitroso-2-naphthol into 825.0 g of water, stir at room temperature until dissolved, and simultaneously open...
Embodiment 3
[0051] Recover and apply mechanically: take 2-naphthol 72.0g and join in 144.0g water (recovered filtrate), stir beating for 30min at room temperature, then add 84.5g of 41% sodium nitrite solution, continue to stir for 10min and pass through pump A ( The flow rate of the raw material liquid is 11mL / min) into the tubular reactor, and at the same time, 20% dilute sulfuric acid is injected into the tubular reactor through pump B (the flow rate is 5mL / min), and the tubular reactor is placed at 0° C. The time was 30min, the reaction solution flowed into the receiving bottle, then filtered at room temperature, the filter cake was washed with water until the Congo red test paper did not change color, and 83.7g of the yellow product was obtained, the yield was 95%, and its content was 98.3% as measured by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com