Preparation method of 2-bromoindolizine derivative
The technology of a derivative, brominzine, is applied in the field of preparation of 2-bromoinzine derivatives, which can solve the problems of increasing the use of reagents and solvents, cumbersome operation steps, and long time consumption, so as to avoid the loss of resources and energy, and the boiling point High, reduce time consumption effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: The preparation method of 2-bromoindolizine derivatives.
[0030] Step 1) In a 25mL round bottom flask, add brominated acetophenonyl-4-cyanopyridine quaternary ammonium salt (2.0mmol), 1-bromoethynyl-4-methylbenzene (1.0mmol), tetramethylpiperidine Pyridine nitrogen oxide (2.0 mmol), potassium carbonate (3.0 mmol) and 2 mL of dimethyl sulfoxide were reacted at 90°C for 2 hours;
[0031] Step 2) After the reaction is over, the resulting mixture is poured into water, extracted, dried, and separated by column chromatography to obtain pure product 3-benzoyl-2-bromo-1-(4-methylphenyl)indazine-7 - Nitrile, structural formula is as follows:
[0032]
[0033] The yield of pure 2-bromoindolizine derivative is 82%, and the product is a yellow solid.
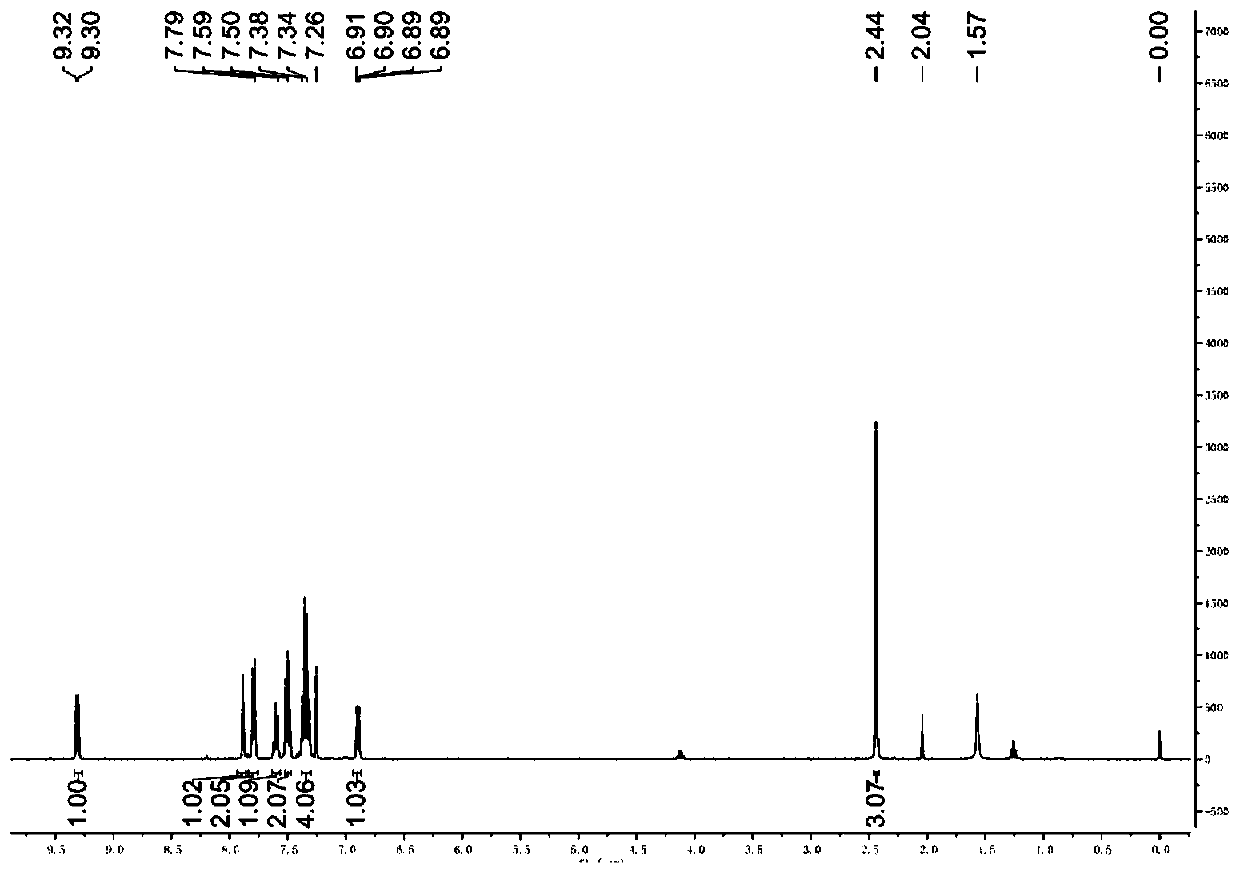
[0034] Such as figure 1 Shown, is the proton nuclear magnetic resonance spectrogram of the 2-bromoinzine derivative pure product that makes in this embodiment: (DMSO-d 6 , 400MHz) (δ, ppm): 9.31(d, J=7.6Hz, 1H),...
Embodiment 2
[0035] Embodiment 2: The preparation method of 2-bromoindolizine derivatives.
[0036] Step 1) In a 25mL round bottom flask, add acetophenonyl bromide-4-cyanopyridinium quaternary ammonium salt (2.0mmol), bromoethynylbenzene (1.0mmol), tetramethylpiperidine nitrogen oxide (2.0mmol ), potassium carbonate (3.0mmol) and dimethyl sulfoxide 2mL, reacted at 90°C for 2 hours;
[0037] Step 2) After the reaction is finished, the resulting mixture is poured into water, extracted, dried, and separated by column chromatography to obtain pure product 3-benzoyl-2-bromo-1-phenylindazine-7-carbonitrile, the structural formula is as follows:
[0038]
[0039] The yield of pure 2-bromoindolizine derivatives prepared by this method is 82%, and the product is a yellow solid.
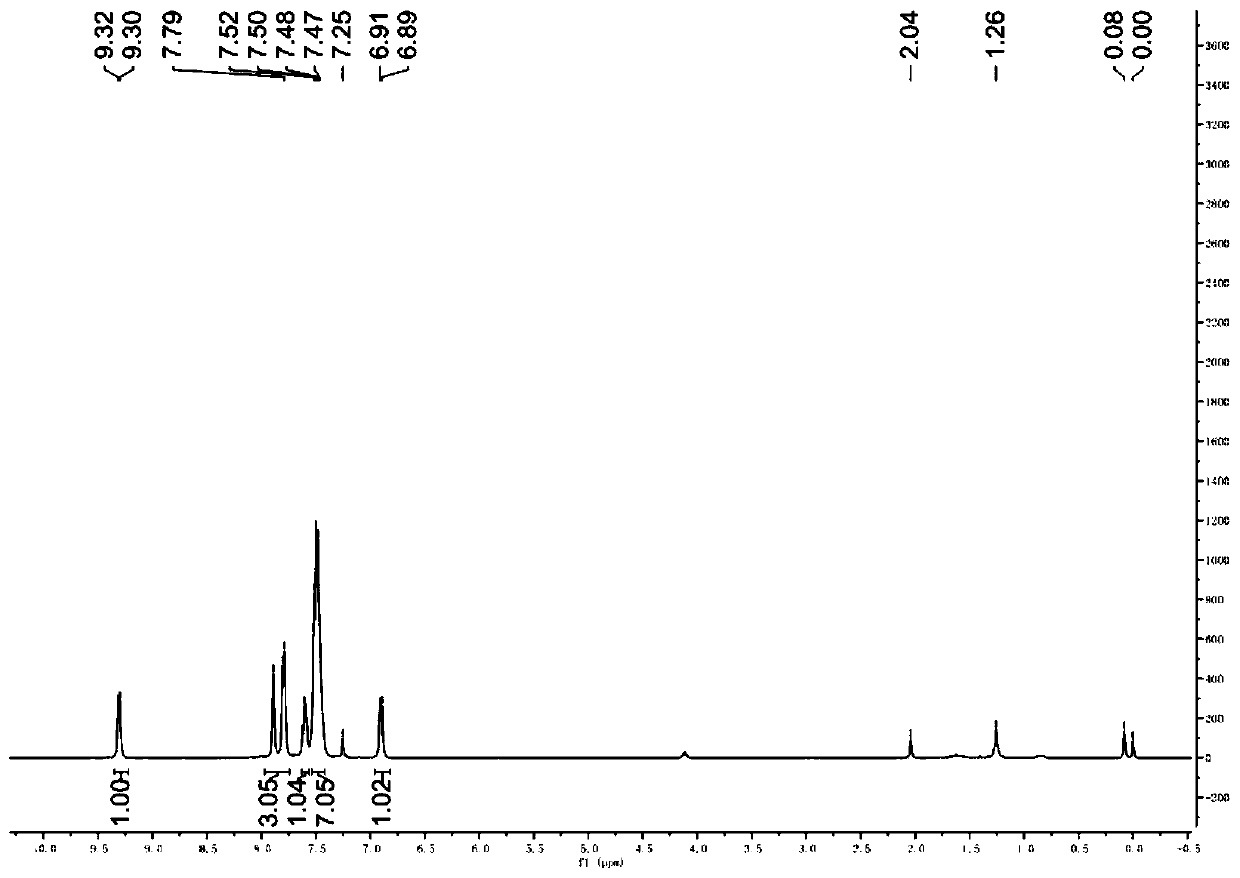
[0040] Such as figure 2 Shown, it is the 2-bromoinzine derivative pure product proton nuclear magnetic resonance spectrogram that makes in this embodiment: (DMSO-d 6 , 400MHz) (δ, ppm): 9.31(d, J=6.8Hz, 1H), 7.89(s,...
Embodiment 3
[0041] Embodiment 3: A kind of preparation method of 2-bromoindolizine derivative.
[0042] Step 1) In a 25mL round bottom flask, add 4-methoxyacetophenonyl-4-cyanopyridine quaternary ammonium bromide (2.0mmol), bromoethynylbenzene (1.0mmol), tetramethylpiperidine nitrogen Oxide (2.0mmol), potassium carbonate (3.0mmol) and 2mL of dimethyl sulfoxide were reacted at 90°C for 2 hours;
[0043] Step 2) After the reaction, the resulting mixture is poured into water, extracted, dried, and separated by column chromatography to obtain pure product 3-(4-methoxybenzoyl)-2-bromo-1-phenylindazine- 7-Nitrile, the structural formula is as follows:
[0044]
[0045] The yield of pure 2-bromoindolizine derivatives obtained by this method is 68%, and the product is a yellow solid.
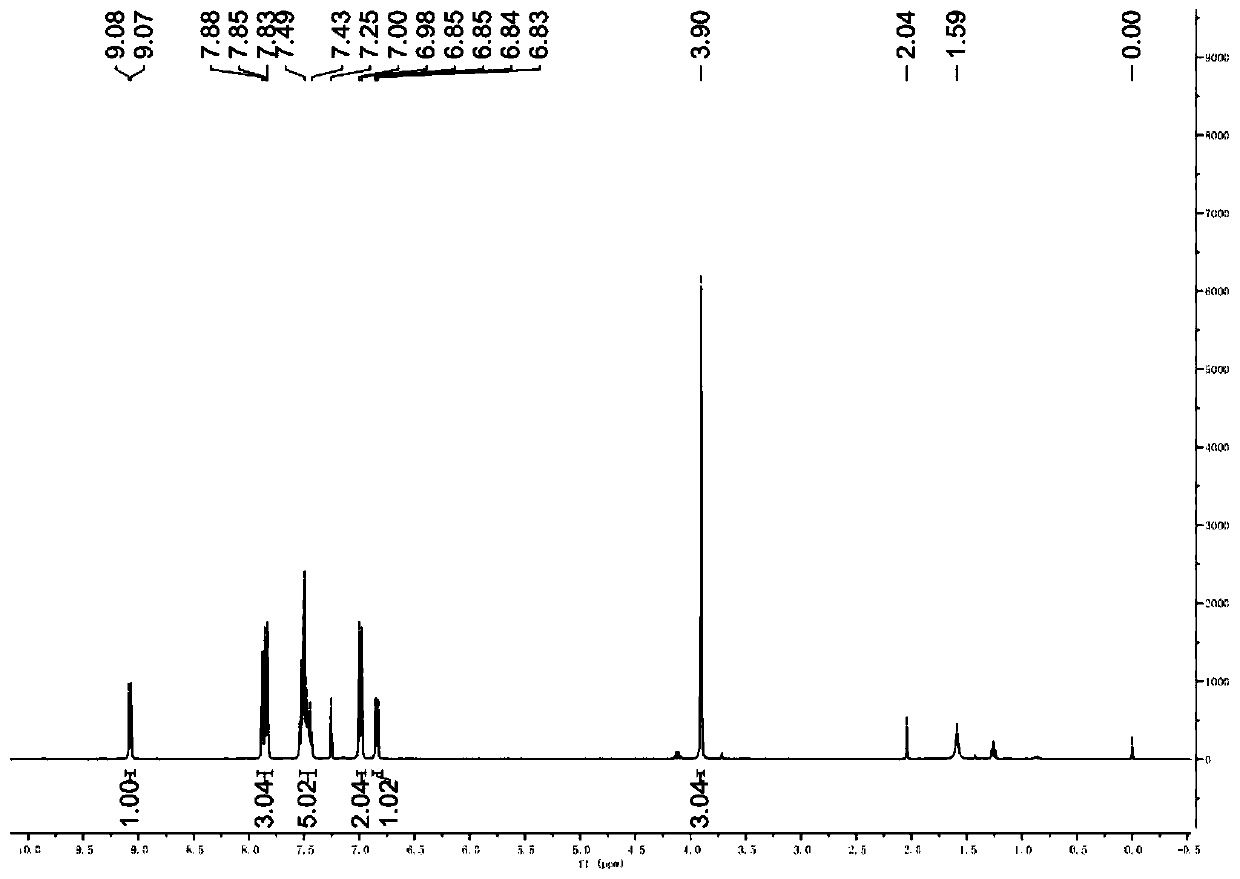
[0046] Such as image 3 Shown, it is the 2-bromoinzine derivative pure product proton nuclear magnetic resonance spectrogram that makes in this embodiment: (DMSO-d 6 , 400MHz) (δ, ppm): 9.08(d, J=7.6Hz, 1H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com