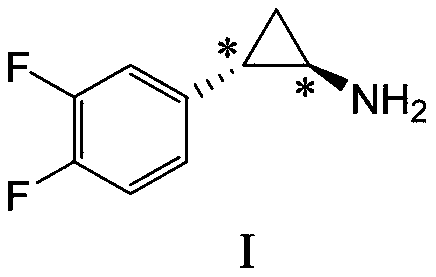
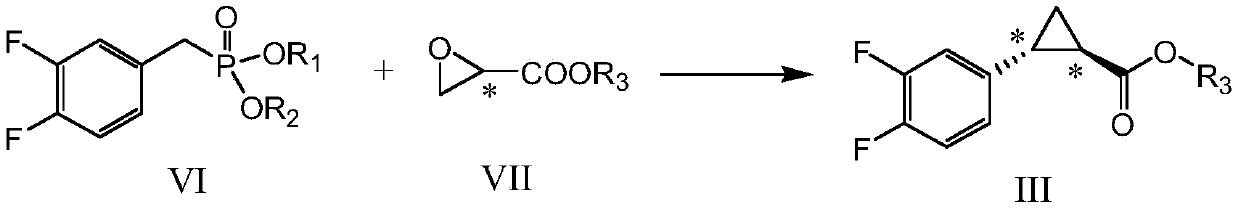Preparation method of chiral aromatic cyclopropylamine and salt thereof and intermediate used in preparation method
A technology of hypochlorite and compound, which is applied to the preparation method of chiral aromatic cyclopropylamine and its salt and the field of intermediates used, can solve the problem of unfriendly environment, low catalytic efficiency of chiral oxazolidinone, and limited production. application and other issues, to achieve the effect of reducing cost, simplifying operation, and high chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
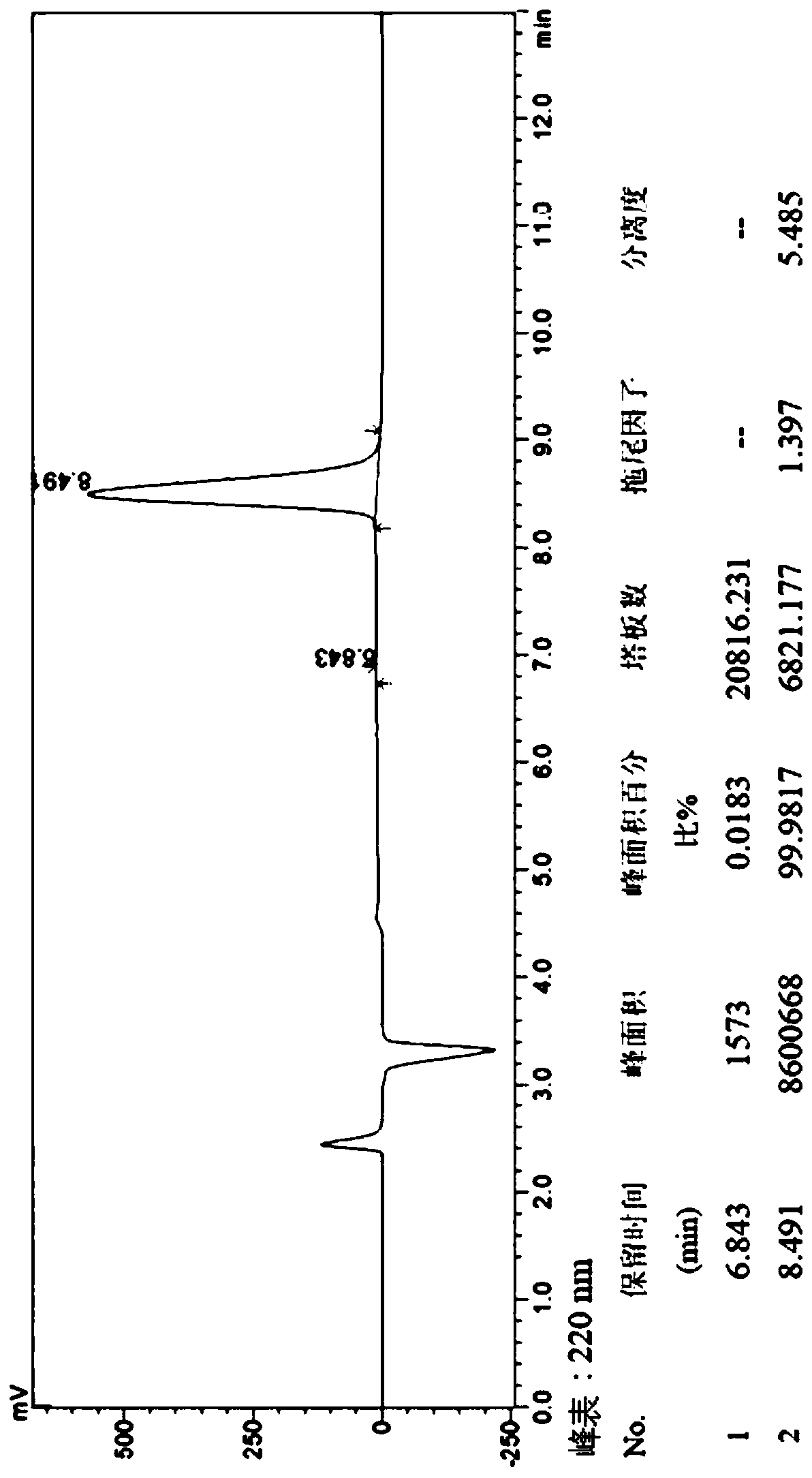
Image
Examples
Embodiment 1
[0067] Example 1: Preparation of 3,4-difluorobenzyl diethyl phosphite (VI-1)
[0068]
[0069] Add triethyl phosphite (25.7g, 0.15mol) and 3,4-difluorobenzyl bromide (27.5g, 0.13mol) into the reaction flask, and heat to 110°C for 20 hours. After the reaction was completed, the reaction was cooled to room temperature, and the excess triethyl phosphite was removed by vacuum concentration to obtain a light yellow oil (VI-1) (31 g, 88.6%), which was directly used in the next reaction.
Embodiment 2
[0070] Example 2: Preparation of 3,4-difluorobenzyl diisopropyl phosphite (VI-2)
[0071]
[0072] Add triisopropyl phosphite (31.2g, 0.15mol) and 3,4-difluorobenzyl bromide (27.5g, 0.13mol) into the reaction flask, and heat to 110°C for 27 hours. After the reaction was completed, it was cooled to room temperature, concentrated under high vacuum and reduced pressure to remove excess triethyl phosphite, and a light yellow oil (VI-2) (27.6 g, 72.6%) was obtained, which was directly used in the next reaction.
Embodiment 3
[0073] Example 3: Preparation of ethyl (1R,2R)-2-(3,4-difluorophenyl)cyclopropanecarboxylate (III-1)
[0074]
[0075] Add 3,4-difluorobenzyl diethyl phosphite (VI-1) (30 g, 0.11 mol) prepared in the previous step into the reaction flask, then add 250 mL of toluene, stir to dissolve, protect with nitrogen, and cool down to 10-15 ℃, add sodium tert-butoxide (11.5g, 0.12mol) in batches, heat up to 50℃ and keep it warm for 2 hours after adding, then cool down to 10-15℃, add R-ethyl glycidate (VII-1) dropwise (12.8g, 0.11mol, chiral purity greater than 99%), the rate of addition was controlled so that the temperature did not exceed 15°C. After the dropwise addition, the temperature was slowly raised to reflux, and the reaction was maintained at reflux for 16 hours. After the reaction, cool down to about 30°C in an ice bath, add 100 mL of water to quench the reaction, separate the toluene layer, extract the water phase with 50 mL of toluene once, combine the organic phases, was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chiral purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com