Heavy metal ion adsorbent and preparation method and application thereof
A technology of heavy metal ions and adsorbents, applied in alkali metal compounds, chemical instruments and methods, adsorption water/sewage treatment, etc., can solve problems such as difficult treatment, secondary damage to water environment, etc., and achieve good repeatability and stability , the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
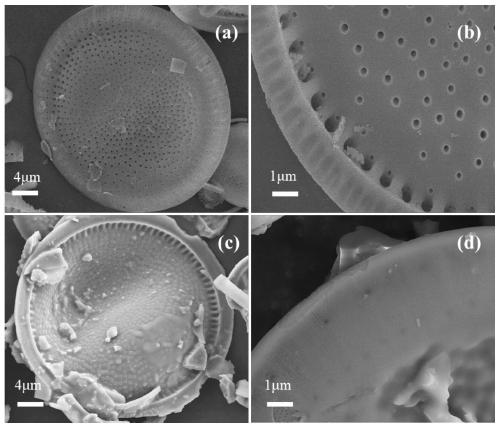
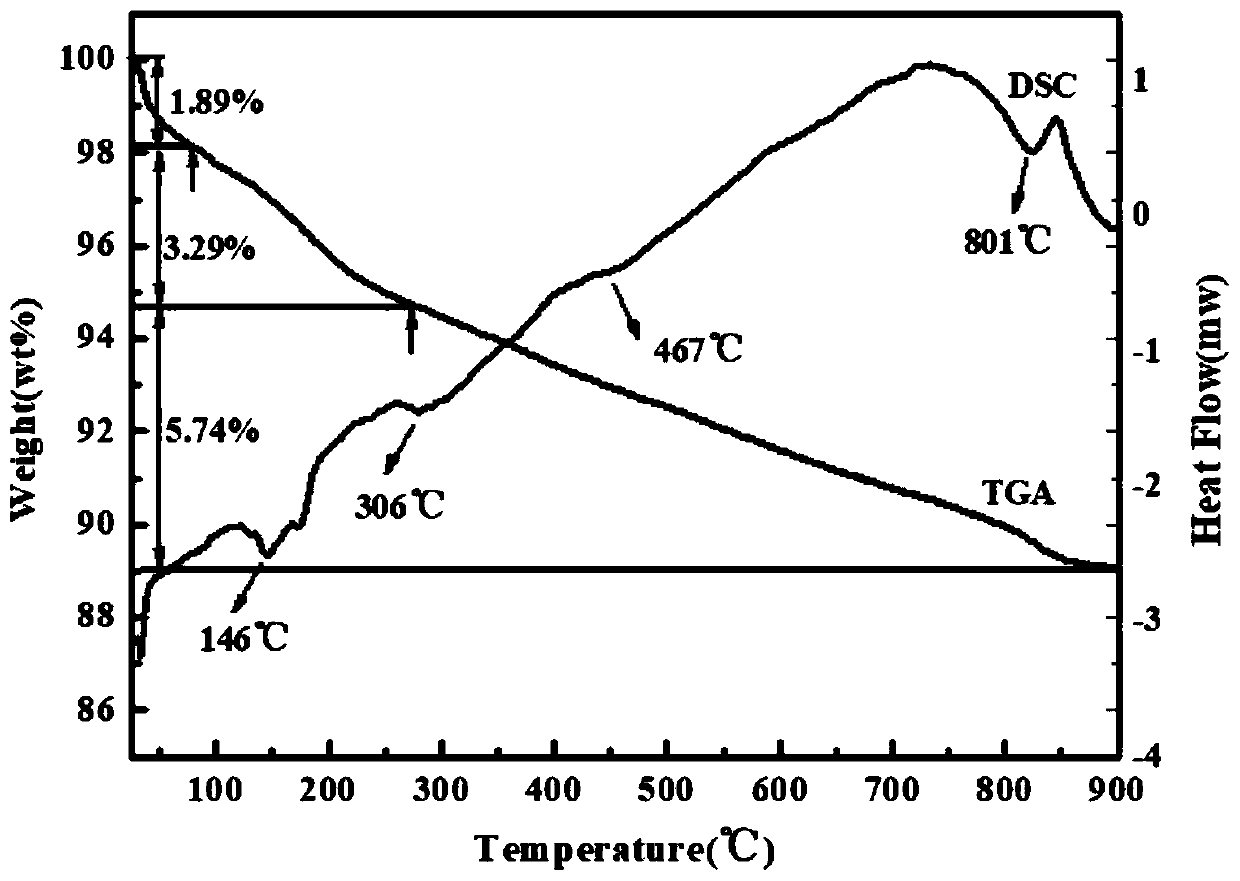
Image
Examples
Embodiment 1
[0046] This embodiment provides a heavy metal ion adsorbent (APTES@diatomite), and the preparation method of the heavy metal ion adsorbent is as follows:
[0047] (1) Add 1 g of diatomite to 100 mL of 0.5mol / L NaOH solution, stir at room temperature for 1 hour, wash and dry to obtain surface-activated diatomite;
[0048] (2) Add the surface-activated diatomite into 50ml ethanol solution, stir at room temperature for 3 minutes, and ultrasonically clean for 3 minutes to obtain a suspension of diatomite;
[0049] (3) Add 0.86mL of 3-aminopropyltriethoxysilane dropwise into the diatomaceous earth suspension, and use acetic acid (CH 3 COOH) to adjust the pH of the system to 6, stir and react in a water bath at 40°C for 24 hours, then filter with suction, wash with ethanol and deionized water three times, and dry.
[0050] The performance detection result of described heavy metal ion adsorbent is as follows:
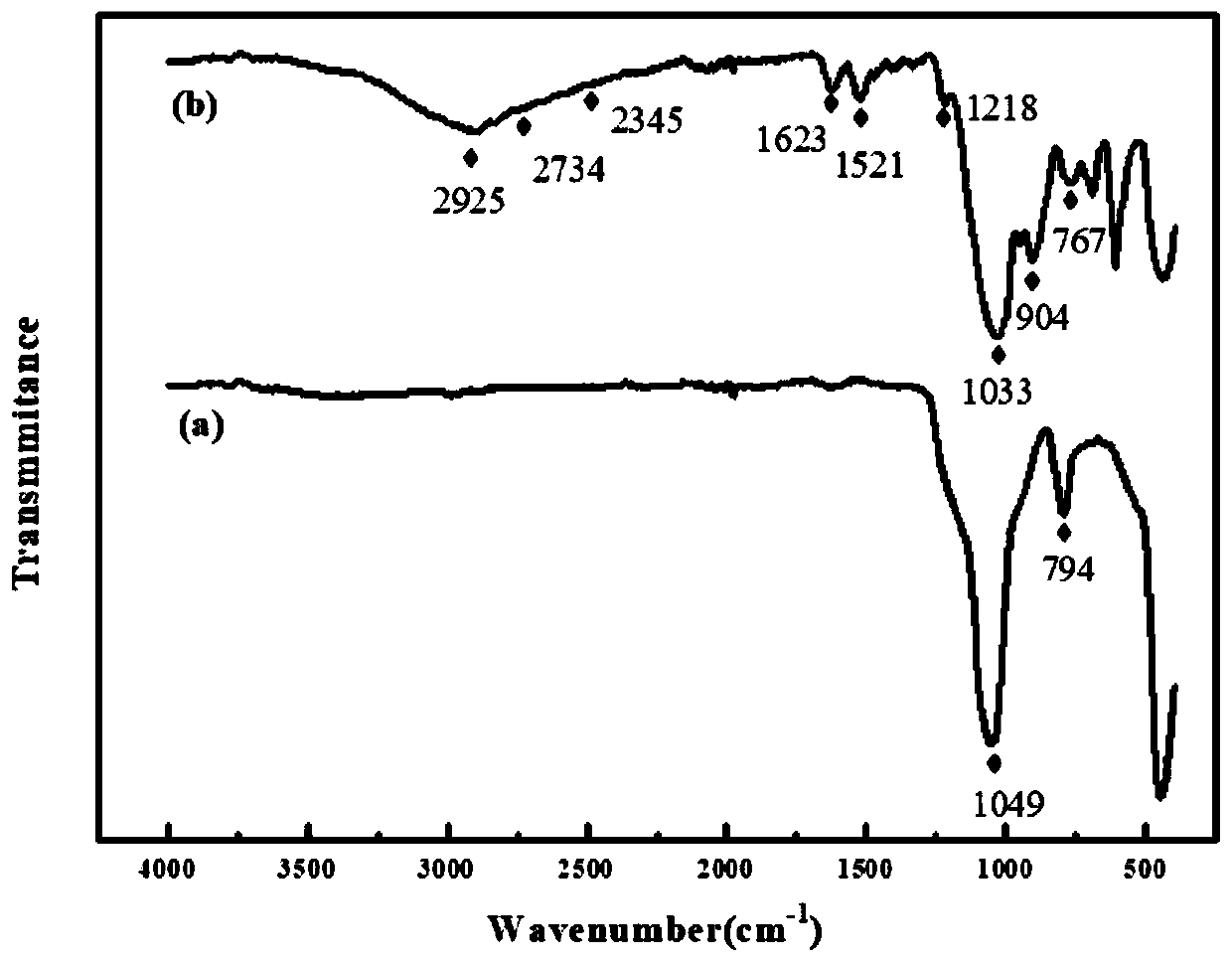
[0051](1) FT-IR image of diatomite and heavy metal ion adsorbent figur...
Embodiment 2
[0056] This embodiment provides a heavy metal ion adsorbent (APTES@diatomite), and the preparation method of the heavy metal ion adsorbent is as follows:
[0057] (1) Add 1 g of diatomite to 100 mL of 0.5mol / L NaOH solution, stir at room temperature for 1 hour, wash and dry to obtain surface-activated diatomite;
[0058] (2) Add the surface-activated diatomite into 50ml ethanol solution, stir at room temperature for 3 minutes, and ultrasonically clean for 3 minutes to obtain a suspension of diatomite;
[0059] (3) Add 1.72 mL of 3-aminopropyltriethoxysilane dropwise into the diatomaceous earth suspension, and use acetic acid (CH 3 COOH) to adjust the pH of the system to 6, reflux and stir at 80°C for 12 hours, filter with suction, wash with ethanol and deionized water three times, and dry.
[0060] Depend on Figure 4 It can be seen that the adsorption capacity of the heavy metal ion adsorbent for Pb(II) is 72.54 mg / g, which is greatly improved compared with diatomite.
Embodiment 3
[0062] This embodiment provides a heavy metal ion adsorbent (APTES@diatomite), and the preparation method of the heavy metal ion adsorbent is as follows:
[0063] (1) Add 1 g of diatomite to 100 mL of 0.5mol / L NaOH solution, stir at room temperature for 1 hour, wash and dry to obtain surface-activated diatomite;
[0064] (2) Add the surface-activated diatomite to 50 mL of toluene solution, stir at room temperature for 3 minutes, and ultrasonically clean for 3 minutes to obtain a suspension of diatomite;
[0065] (3) Add 0.86mL of 3-aminopropyltriethoxysilane dropwise to the diatomaceous earth suspension, use hydrochloric acid (HCl) to adjust the pH of the system to 6, reflux and stir at 80°C for 12 hours, then suction filter, Wash three times with ethanol and deionized water respectively, and dry.
[0066] Depend on Figure 4 It can be seen that the adsorption capacity of the heavy metal ion adsorbent for Pb(II) is 57.6 mg / g, which is greatly improved compared with diatomite...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
| Adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com