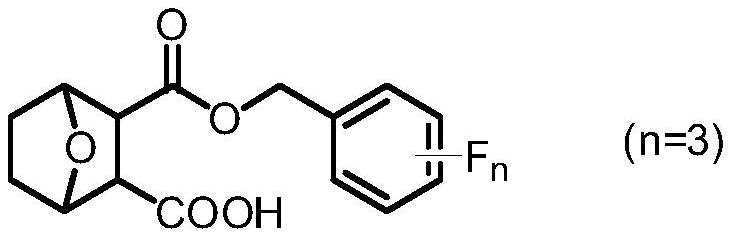
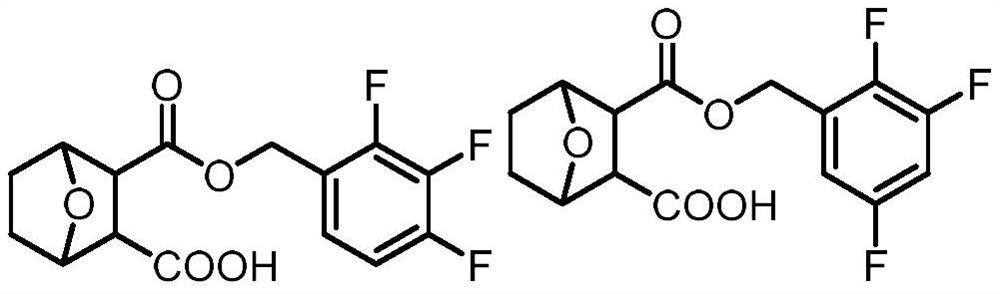
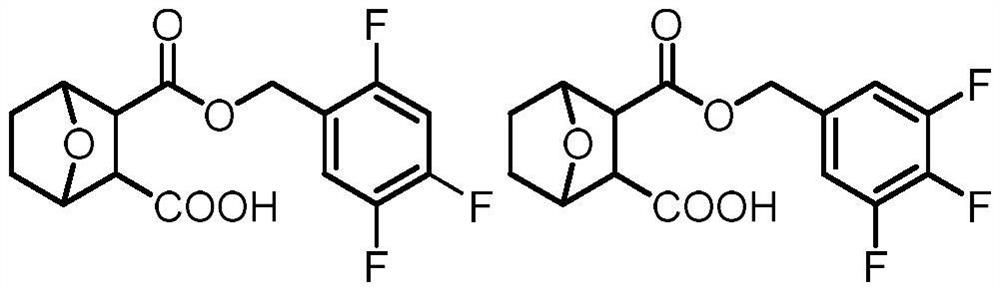
Norcantharidin carboxylate trifluorobenzyl ester and its synthesis method and application
A technology of cantharidin carboxylic acid and trifluorobenzyl ester, which is applied in the field of medicine to achieve the effects of improving water solubility, readily available raw materials, and easy operation and implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of 5-enenorcantharidin 3:
[0040] Take 2.6 g of maleic anhydride 1 (26 mmol), put it in a round-bottomed flask, add 20 mL of diethyl ether to the flask to completely dissolve the maleic anhydride, and after it is completely dissolved, slowly add furan 22.9 mL (39 mmol) dropwise, The reaction was carried out at room temperature for 24 h, filtered with suction, and dried to obtain a white solid compound 3, namely 5-enenorcantharidin, which was dried and weighed to 2.1 g, and the yield was 48%. 1 HNMR(400Hz, DMSO-d 6 ): δ6.58(s, 2H), 5.35(s, 2H), 3.31(d, J=4.0Hz, 2H).
[0041] In addition to diethyl ether, the organic solvent used for dissolving maleic anhydride in step 1 of the above embodiment 1 can also be replaced by any one of dichloromethane, chloroform and tetrahydrofuran.
Embodiment 2
[0043] Preparation of Norcantharidin 4:
[0044]At room temperature, the white solid compound 3 (10mmol, 1.7g) obtained in step 1 was taken, and 10% palladium carbon (0.16g, 15mmol%) was successively added to a 100mL three-necked flask, plugged and evacuated, passed into hydrogen, and then added with acetic acid Ethyl ester 30mL, stirred at room temperature for 24h. After the reaction is completed, suction filtration, the filter cake is washed with ethyl acetate for 2-3 times, and the obtained filtrate is concentrated under reduced pressure to obtain white solid intermediate 4 (1.6 g), namely norcantharidin, with a yield of 97%. 1 HNMR(400Hz, DMSO-d 6 ): 4.86(s, 2H), 3.39(s, 2H), 1.65(s, 4H).
Embodiment 3
[0046] Preparation of norcantharidin carboxylate-2,3,4-trifluorobenzyl ester (compound 6-1):
[0047]
[0048] Norcantharidin 4 (1.0 mmol, 168 mg) and 4-DMAP (1.0 mmol, 244 mg) were added to a 25 mL sealed tube, and after three times of argon replacement, 2.5 mL of DCM, 2,3,4-trifluorocarbon were added successively. Benzyl alcohol 5 (2.0 mmol, 232 μL) was reacted at 60 °C for 14 h. After the reaction was completed, it was cooled to room temperature, washed three times with HCl (1mol / L) and saturated brine respectively, and the organic phases were combined. After the organic phases were dried with anhydrous sodium sulfate, the white solid product 6-1 was obtained by flash column chromatography. The yield was was 33.5%. 1 H NMR (400MHz, DMSO-d 6 )δ12.26(s,1H),7.37-7.27(m,2H),5.09(d,J=12.8Hz,1H),4.97(d,J=12.8Hz,1H),4.69(d,J=2.4 Hz,2H),3.04(s,2H),1.60-1.50(m,4H). 19 F NMR (376MHz, DMSO-d 6 )δ-139.35,-143.11(d,J=15.0Hz,1F),-166.16(t,J=18.8Hz,1F).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com