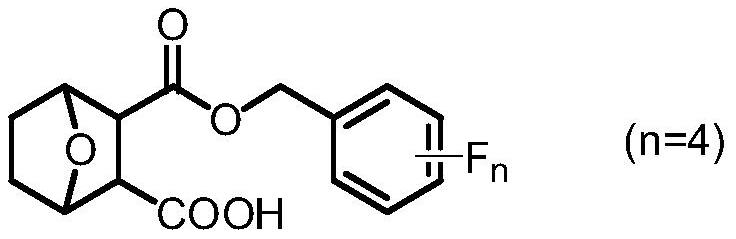
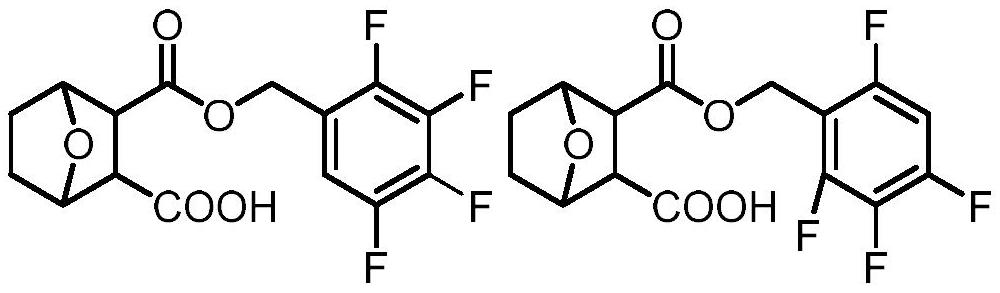
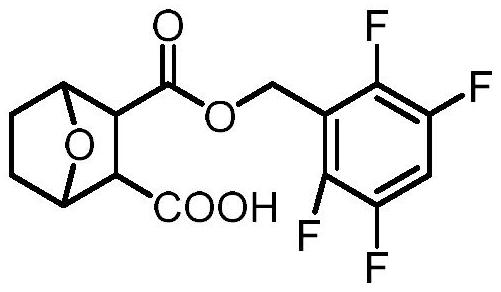
Norcantharidin carboxylate tetrafluorobenzyl ester and synthesis method thereof
A technology of tetrafluorobenzyl methylcantharidin carboxylate and methylcantharidin carboxylic acid, which is applied in the field of medicine to achieve the effects of enhancing pharmacokinetic efficacy, readily available raw materials, and easy operation and implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The following will further illustrate the present invention through specific examples, which are not intended to limit the protection scope of the present invention. On the premise of not departing from the concept of the present invention, those skilled in the art can make improvements or combinations to the parameters or conditions of the claims, and these improvements or combinations should also be regarded as the protection scope of the present invention. Therefore, the protection scope of the patent of the present invention should be subject to the appended claims.
[0033] Furan, maleic anhydride, 2,3,4,5-tetrafluorobenzyl alcohol, 2,3,4,6-tetrafluorobenzyl alcohol, 2,3,5,6-tetrafluorobenzene used in the present invention Methanol was from Saen Chemical Technology (Shanghai) Co., Ltd.; the solvent used was from Shanghai Titan Technology Co., Ltd. Unless otherwise specified, all reagents used are chemically pure.
[0034] The synthetic method of the aforementione...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com