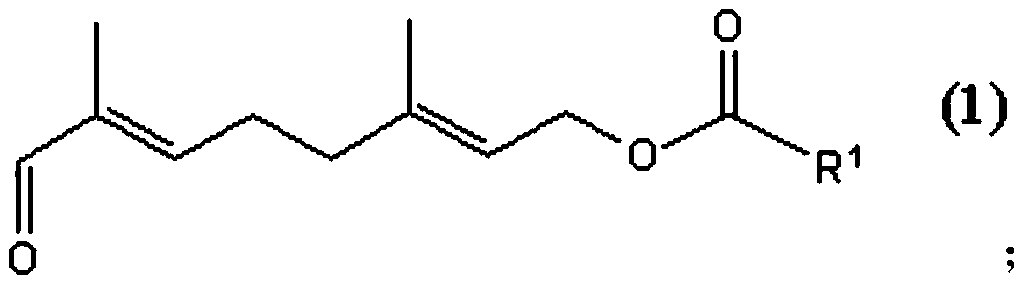
Purification method of 8-oxo-3, 7-dimethyl-octadiene carboxylic ester compound
A technology of octadienyl carboxylate and purification method, which is applied in the separation/purification of carboxylate, the preparation of organic compounds, the preparation of carboxylate, etc., can solve problems such as being unsuitable for large-scale production, and achieve a simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 45.0g crude 8-oxo-3,7-dimethyl-octadienyl formate compound (content of 8-oxo-3,7-dimethyl-octadienyl formate (cis Formula + trans): 67.5%) is miscible with 50g of methanol, and then added dropwise to 69.0g of 35% sodium bisulfite aqueous solution with a mass percentage of 69.0g, the temperature was kept at 60°C, the dropwise addition was completed in 0.5h, and then reacted for 4h , The reaction of 8-oxo-3,7-dimethyl-octadienyl formate was complete by gas phase detection. Recover methanol by distillation from the reaction mixture, extract the organic phase three times with 120ml dichloromethane, and separate The aqueous layer of sodium sulfonate, in the gained aqueous layer, add the dichloromethane of 100ml, at normal temperature, slowly add dropwise to wherein the mass percentage of 101.7g be 10% hydrochloric acid aqueous solution, the gained reaction mixture is stirred at normal temperature for 1 hours to react. After the reaction was completed, the reaction mixture ...
Embodiment 2
[0040] 40.0g crude 8-oxo-3,7-dimethyl-octadienyl propionate compound (8-oxo-3,7-dimethyl-octadienyl propionate content (cis Formula + trans): 62.5%) is miscible with 50g of methanol, then added dropwise to 49.7g of 35% sodium bisulfite aqueous solution with a mass percentage of 35%, the temperature was kept at 60°C, the dropwise addition was completed in 0.5h, and then reacted for 4h , The reaction of 8-oxo-3,7-dimethyl-octadienyl propionate was completed by gas phase detection. Recover methanol by distillation from the reaction mixture, extract the organic phase three times with 120ml dichloromethane, and separate the The aqueous layer of sodium sulfonate, in the gained aqueous layer, add the dichloromethane of 100ml, at normal temperature, to wherein dropwise add 33.2g of the mass percent of 20% sodium hydroxide aqueous solution, the gained reaction mixture is stirred at normal temperature 1 hour for reaction. After the reaction was complete, the pH of the aqueous layer wa...
Embodiment 3
[0042] The crude 8-oxo-3,7-dimethyl-octadienyl acetate compound of 70.0g (the content of 8-oxo-3,7-dimethyl-octadienyl acetate (cis Formula + trans): 65.0%) is miscible with 80g of methanol, and then added dropwise to 97.0g of 35% aqueous sodium bisulfite solution with a mass percentage of 97.0g, the temperature was kept at 60°C, the dropwise addition was completed in 0.5h, and then reacted for 4h , gas phase detection of 8-oxo-3,7-dimethyl-octadienyl acetate was completely reacted, and methanol was recovered by distillation from the reaction mixture. Use 150ml dichloromethane to extract the organic phase three times, separate the aqueous layer containing α-hydroxyl [8-acetoxyl-2,6-dimethyl-2,6-octadienyl] sodium sulfonate, and add to the obtained 150ml of dichloromethane was added to the water layer, and 64.9g of 20% by mass sodium hydroxide aqueous solution was added dropwise thereto at room temperature, and the resulting reaction mixture was stirred at room temperature for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com