Compound preparation for preventing transplant rejection after renal transplantation
A technology of transplant rejection and compound preparations, which is applied in the field of compound preparations for the prevention of transplant rejection after kidney transplantation, can solve problems such as thrombocytopenia, achieve the effects of reducing side effects, improving thrombocytopenia, and relieving headaches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
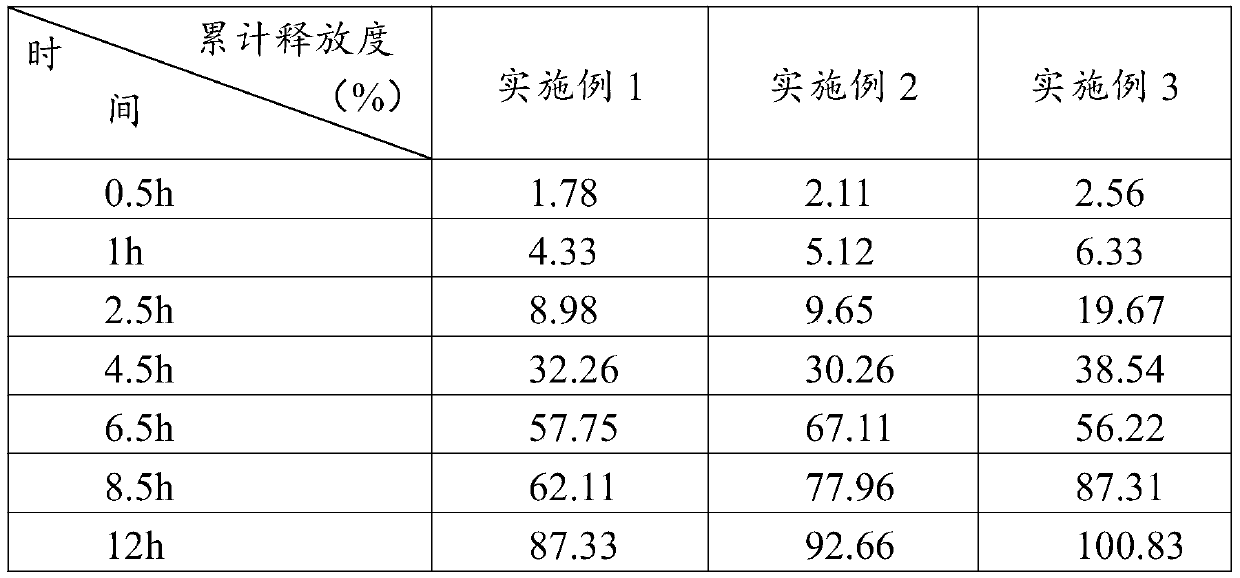
Embodiment 1
[0027] This embodiment discloses a compound preparation for preventing transplant rejection after kidney transplantation, including the following components in parts by weight:
[0028] Component weight Tacrolimus5g mixture30g Methyl cellulose: Claw ear gum = 1:3 (weight ratio)30g Corn starch: MCC101=3:1 (weight ratio)30g Povidone K304g Magnesium stearate 1g
[0029] In this case, the preparation method of the compound preparation for preventing transplant rejection after kidney transplantation includes the following steps:
[0030] S1. Put tacrolimus into a jet mill for micronization. D90 is required to be 5-12μm.
[0031] S2. The mixture is composed of white peony root, Chuanqiong, Taizi ginseng, licorice, Ziheche, Yinchen, and Cnidium in any proportion, and is made into very fine powder by a fine powder grinder.
[0032] S3. Weigh the prescribed amount of sustained-release materials, fillers, binders, and lubricants, and pass them through an 80-mesh sieve for use.
[0033] S...
Embodiment 2
[0041] This embodiment discloses a compound preparation for preventing transplant rejection after kidney transplantation, including the following components in parts by weight:
[0042] Component weight Tacrolimus8g mixture30g Methyl cellulose: Claw ear gum = 1:3 (weight ratio)24g Corn starch: MCC101=3:1 (weight ratio)33g Povidone K304g Magnesium stearate 1g
[0043] In this case, the preparation method of the compound preparation for preventing transplant rejection after kidney transplantation includes the following steps:
[0044] S1. Put tacrolimus into a jet mill for micronization. D90 is required to be 5-12μm.
[0045] S2. The mixture is composed of white peony root, Chuanqiong, Taizi ginseng, licorice, Ziheche, Yinchen, and Cnidium in any proportion, and is made into a very fine powder by a fine powder grinder.
[0046] S3. Weigh the prescribed amount of sustained-release materials, fillers, binders, and lubricants, and pass them through an 80-mesh sieve for use.
[0047]...
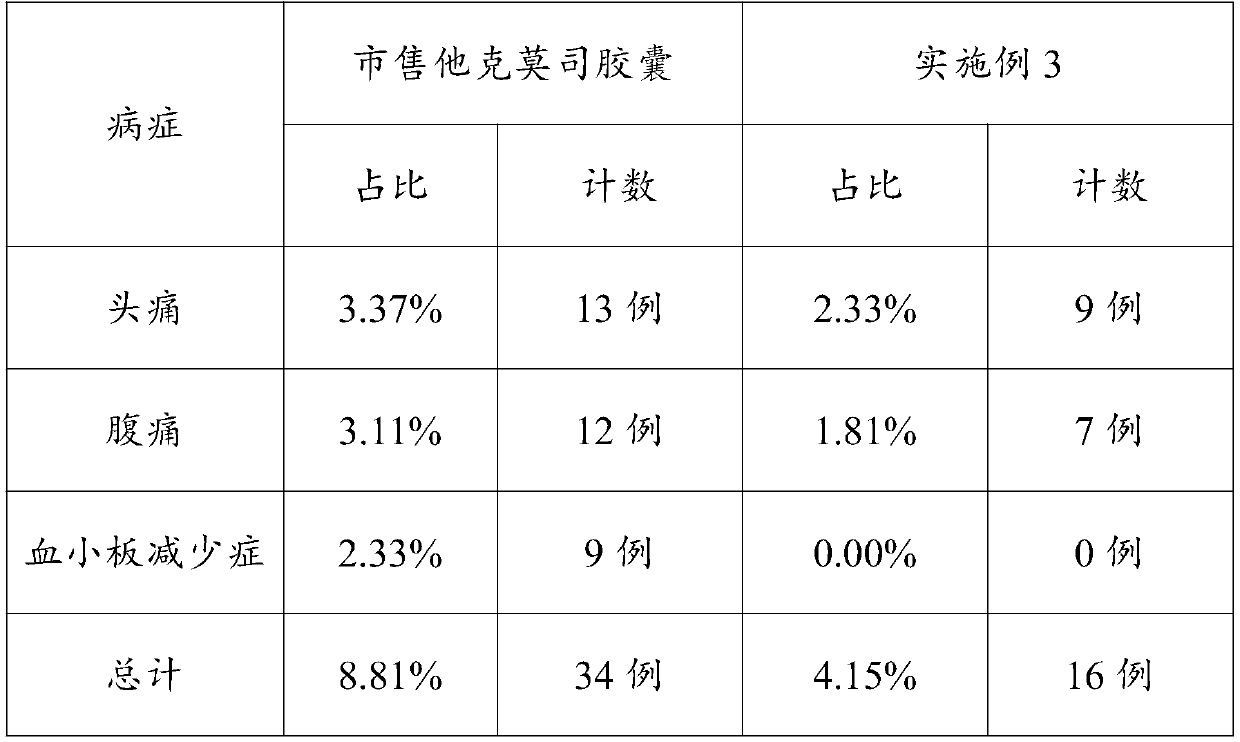
Embodiment 3
[0055] This embodiment discloses a compound preparation for preventing transplant rejection after kidney transplantation, including the following components in parts by weight:
[0056] Component weight Tacrolimus10g mixture25g Methyl cellulose: Claw ear gum = 1:3 (weight ratio)20g Corn starch: MCC101=3:1 (weight ratio)36g Povidone K306g Magnesium stearate 3g
[0057] The preparation method includes the following steps:
[0058] S1. Put tacrolimus into a jet mill for micronization. D90 is required to be 5-12μm.
[0059] S2. The mixture is composed of white peony root, Chuanqiong, Taizi ginseng, licorice, Ziheche, Yinchen, and Cnidium in any proportion, and is made into very fine powder by a fine powder grinder.
[0060] S3. Weigh the prescribed amount of sustained-release materials, fillers, binders, and lubricants, and pass them through an 80-mesh sieve for use.
[0061] S4. Prepare the adhesive with 70% ethanol as a solvent to prepare an 8% adhesive solution for later use.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com