Preparation method of DL-p-methylsulfonylphenylserine ester
A technology of methylsulfonyl phenylserine ester and methylsulfonyl phenylserine, which is applied in the field of drug synthesis, can solve the problems of low production capacity, long recovery cycle and the like, and achieves the effects of simplifying production process, improving efficiency and reducing pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
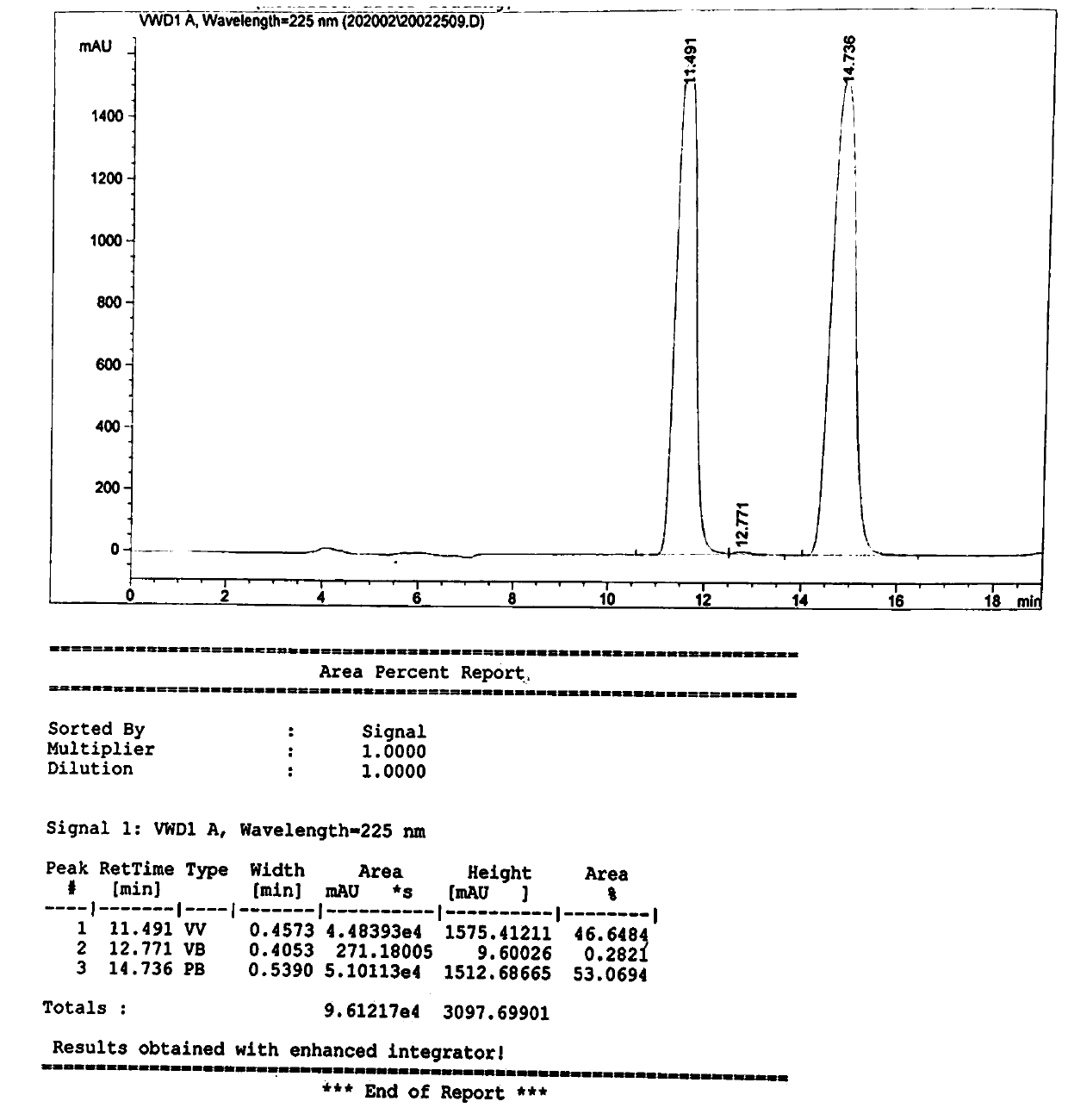
[0037] Take 28.7g (0.1mol) of L-p-thymphenylphenylserine ethyl ester, disperse it into 170g of water, control the temperature at 15°C (temperature difference ±2°C), keep stirring for 30 minutes, then add hydrogen with a concentration of 30wt% Sodium oxide solution 3.9g, heat preservation reaction for 8 hours, then adjust the pH value to 7.0, and then filter to obtain a total of 18.52g of the product D-p-thiamphenyl phenylserine ethyl ester and L-p-thiamphenyl phenylserine ethyl ester. Such as figure 1 As shown, the characteristic peak at 11.491min is the characteristic peak of D-p-thymphenyl phenylserine ethyl ester, and the characteristic peak at 14.736min is the characteristic peak of L-p-thymphenyl phenylserine ethyl ester. In the product, D-p-methyl The ratio of sulfonyl phenylserine ethyl ester to L-p-thiamphenyl phenylserine ethyl ester is 46.6%:53.1%, which is 1:1.1, which is close to 1:1.
Embodiment 2
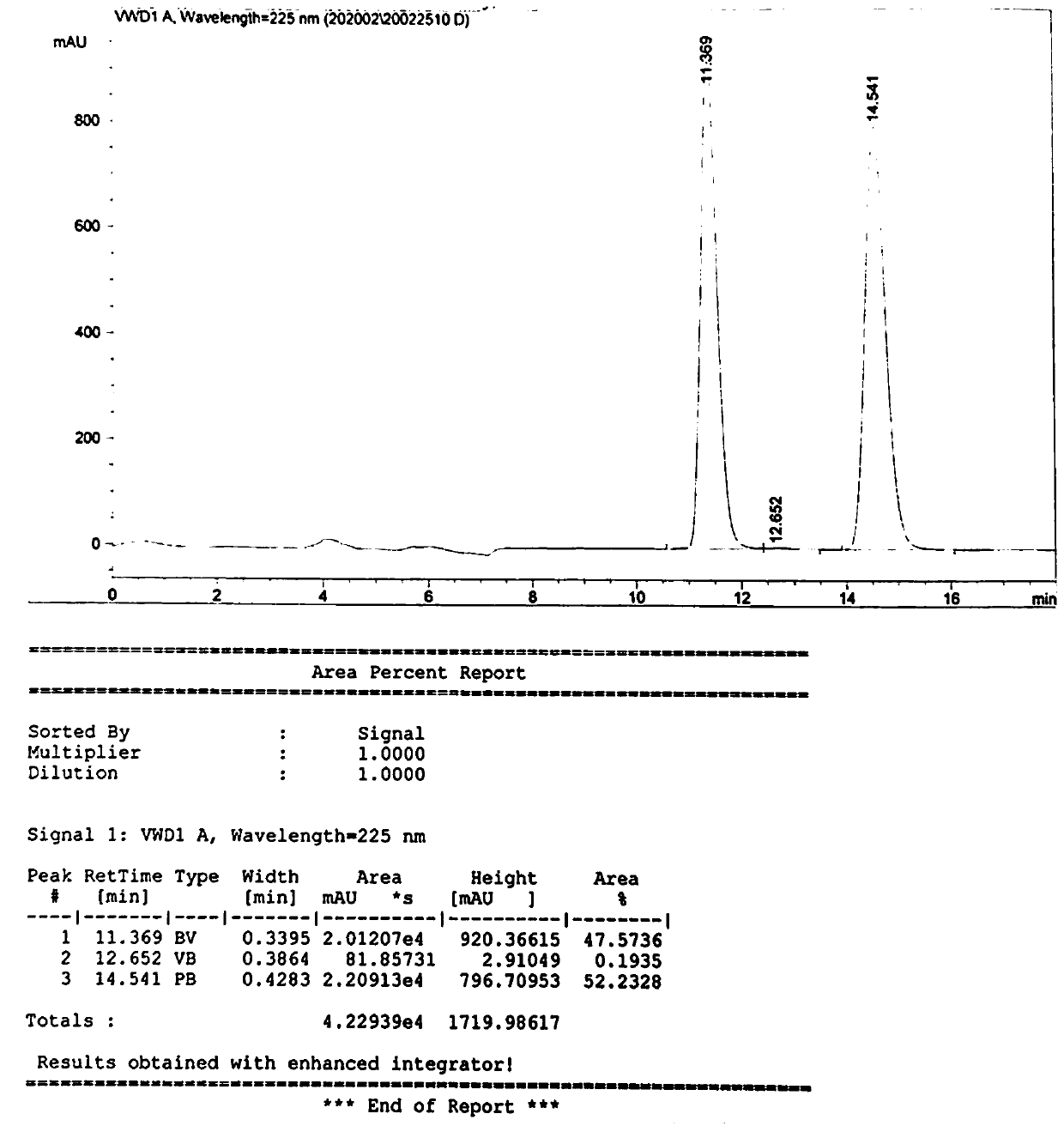
[0039] Take 28.7g (0.1mol) of L-p-thymphenylphenylserine ethyl ester, disperse it into 170g of ethanol, control the temperature at 15°C (temperature difference ±2°C), keep stirring for 30 minutes, and then add a concentration of 10% hydroxide 11.5 g of sodium solution was incubated for 10 hours, then the pH value was adjusted to 7.0, and 15.32 g of the product D-p-thiamphenylphenylserine ethyl ester and L-p-thiamphenyl phenylserine ethyl ester were obtained by filtration. Such as figure 2 As shown, the characteristic peak at 11.369min is the characteristic peak of D-p-thymphenyl phenylserine ethyl ester, and the characteristic peak at 14.541min is the characteristic peak of L-p-thymphenyl phenylserine ethyl ester. In the product, D-p-methyl The ratio of sulfonyl phenylserine ethyl ester to L-p-thiamphenyl phenylserine ethyl ester is 47.6%:52.2%, which is 1:1.1, which is close to 1:1.
Embodiment 3
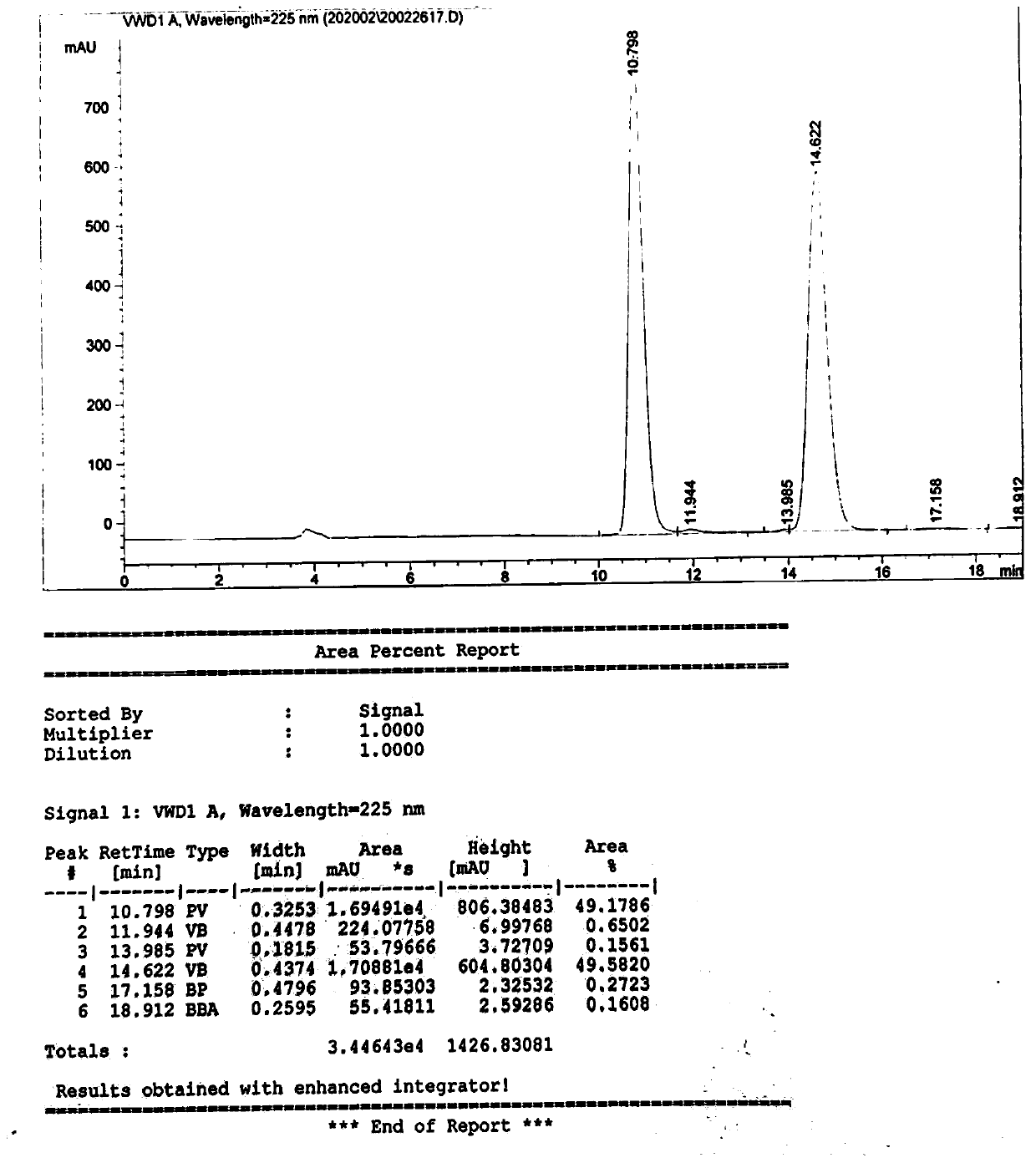
[0041] Take 28.7g (0.1mol) of L-p-thymphenylphenylserine ethyl ester, disperse it in 170g of methanol, control the temperature at 15°C (temperature difference ±2°C), keep stirring for 30 minutes, then use 10% sodium hydroxide solution 11.5 g, heat preservation reaction for 10 hours, then adjust the pH value to 7.0, and filter to obtain a total of 13.32 g of the product D-p-thiamphenyl phenylserine ethyl ester and L-p-thiamphenyl phenyl serine ethyl ester. Such as image 3 As shown, the characteristic peak at 10.798min is the characteristic peak of D-p-thymphenyl phenylserine ethyl ester, and the characteristic peak at 14.622min is the characteristic peak of L-p-thymphenyl phenylserine ethyl ester. In the product, D-p-methyl The ratio of sulfonyl phenylserine ethyl ester to L-p-thiamphenyl phenylserine ethyl ester is 49.2%:49.6%, which is 1:1.0, which is close to 1:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com