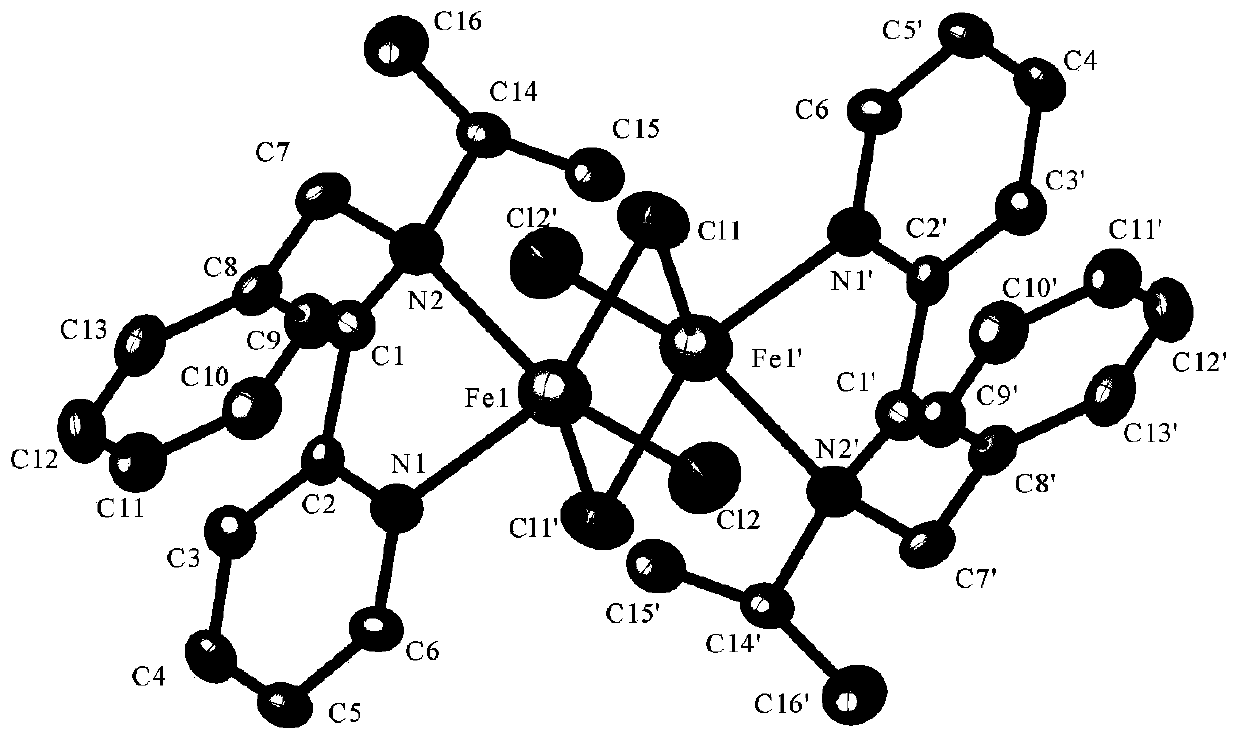
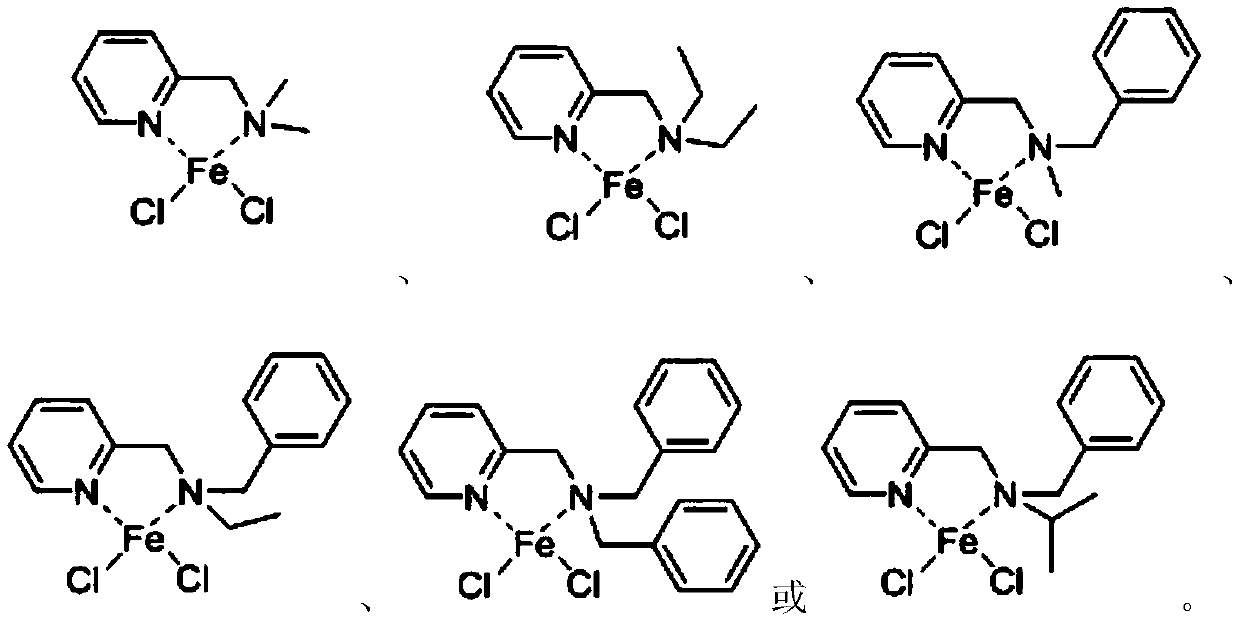
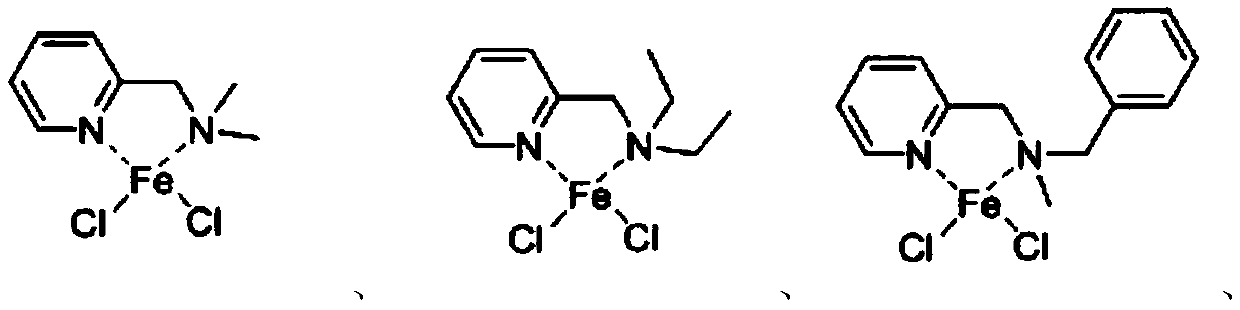
Pyridine tertiary amine iron complex and preparation method thereof, and method for catalyzing polymerization of conjugated diene by using pyridine tertiary amine iron complex
A technology of pyridine tertiary amine iron and conjugated diene, which is applied in the direction of iron-organic compounds, can solve the problems of lower catalyst activity and selectivity, poor heat resistance of iron-based catalysts, and change, and achieve low cost and high molecular weight , Preparation is simple and easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0051] Specific embodiment one: the structural formula of a kind of pyridine tertiary amine iron complex of the present embodiment is:
[0052]
[0053] Its preparation method is as follows: under argon atmosphere, 25mL Schlenk tube was pumped and baked three times, and then 10mL redistilled dichloromethane, 1.0mmol anhydrous FeCl 2and 1.0 mmol of pyridine tertiary amine ligand L1, stirred at room temperature for 24 hours, after the reaction was completed, filtered under an argon atmosphere, vacuum-dried dichloromethane, and then washed twice with 10 mL redistilled n-hexane until the filtrate was clear, and then vacuum-pumped Dry to constant weight to obtain a yellow solid, that is, pyridine tertiary amine iron complex (referred to as catalyst 1) (217.2 mg, yield: 83%).
[0054] Mass Spectrometry: C 16 h 24 Cl 4 Fe 2 N 4: [M-FeCl 2 +H] + : Theoretical value: 399.0806; measured value: 399.0807.
[0055] Elemental Analysis: C 16 h 24 Cl 4 Fe 2 N 4 : Theoretical v...
specific Embodiment approach 2
[0056] Specific embodiment two: the structural formula of a kind of pyridine tertiary amine iron complex of the present embodiment is:
[0057]
[0058] Its preparation method is as follows: under an argon atmosphere, a 25mL Schlenk tube was pumped and baked three times, and then 10mL redistilled dichloromethane, 1.0mmol anhydrous FeCl 2 and 1.0 mmol pyridine tertiary amine ligand L2, stirred at room temperature for 24 hours, after the reaction was completed, filtered under argon atmosphere, vacuum-dried dichloromethane, and then washed twice with 10 mL redistilled n-hexane until the filtrate was clear, and then vacuum-pumped Dry to constant weight to obtain a yellow solid, that is, pyridine tertiary amine iron complex (referred to as catalyst 2) (237.8 mg, yield: 82%).
[0059] Mass Spectrometry: C 20 h 32 Cl 4 Fe 2 N 4: [M-FeCl 3 ] + : Theoretical value: 419.1665; measured value: 419.1663.
[0060] Elemental Analysis: C 20 h 32 Cl 4 Fe 2 N 4 : Theoretical val...
specific Embodiment approach 3
[0061] Specific embodiment three: the structural formula of a kind of pyridine tertiary amine iron complex of the present embodiment is:
[0062]
[0063] Its preparation method is as follows: under an argon atmosphere, a 25mL Schlenk tube was pumped and baked three times, and then 10mL redistilled dichloromethane, 1.0mmol anhydrous FeCl 2 and 1.0 mmol of pyridine tertiary amine ligand L3, stirred at room temperature for 24 hours, after the reaction was completed, filtered under an argon atmosphere, vacuum-dried dichloromethane, and then washed twice with 10 mL redistilled n-hexane until the filtrate was clear, and then vacuum-pumped Dry to constant weight to obtain a yellow solid, ie pyridine tertiary amine iron complex (referred to as Catalyst 3) (272.2 mg, yield: 81%).
[0064] Mass Spectrometry: C 28 h 32 Cl 4 Fe 2 N 4: [M-FeCl 3 ] + : Theoretical value: 515.1665; measured value: 515.1666.
[0065] Elemental Analysis: C 28 h 32 Cl 4 Fe 2 N 4 : Theoretical v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com