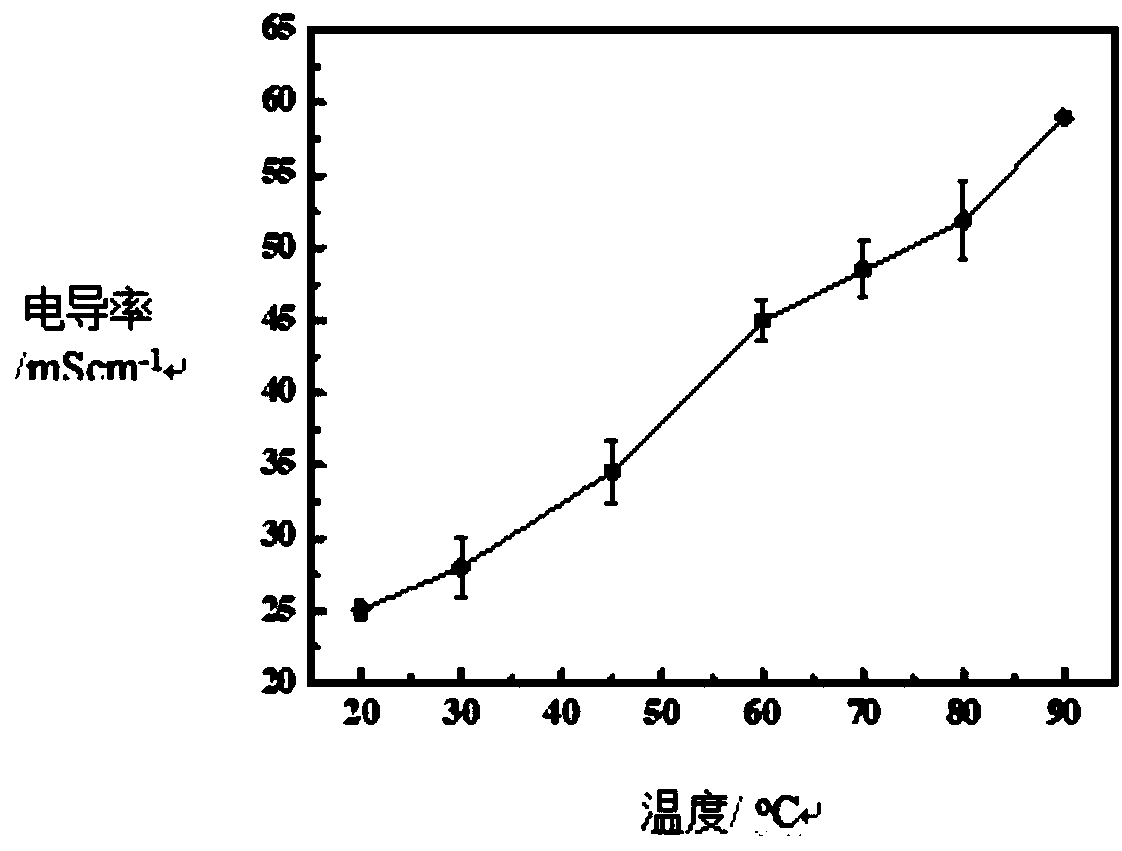
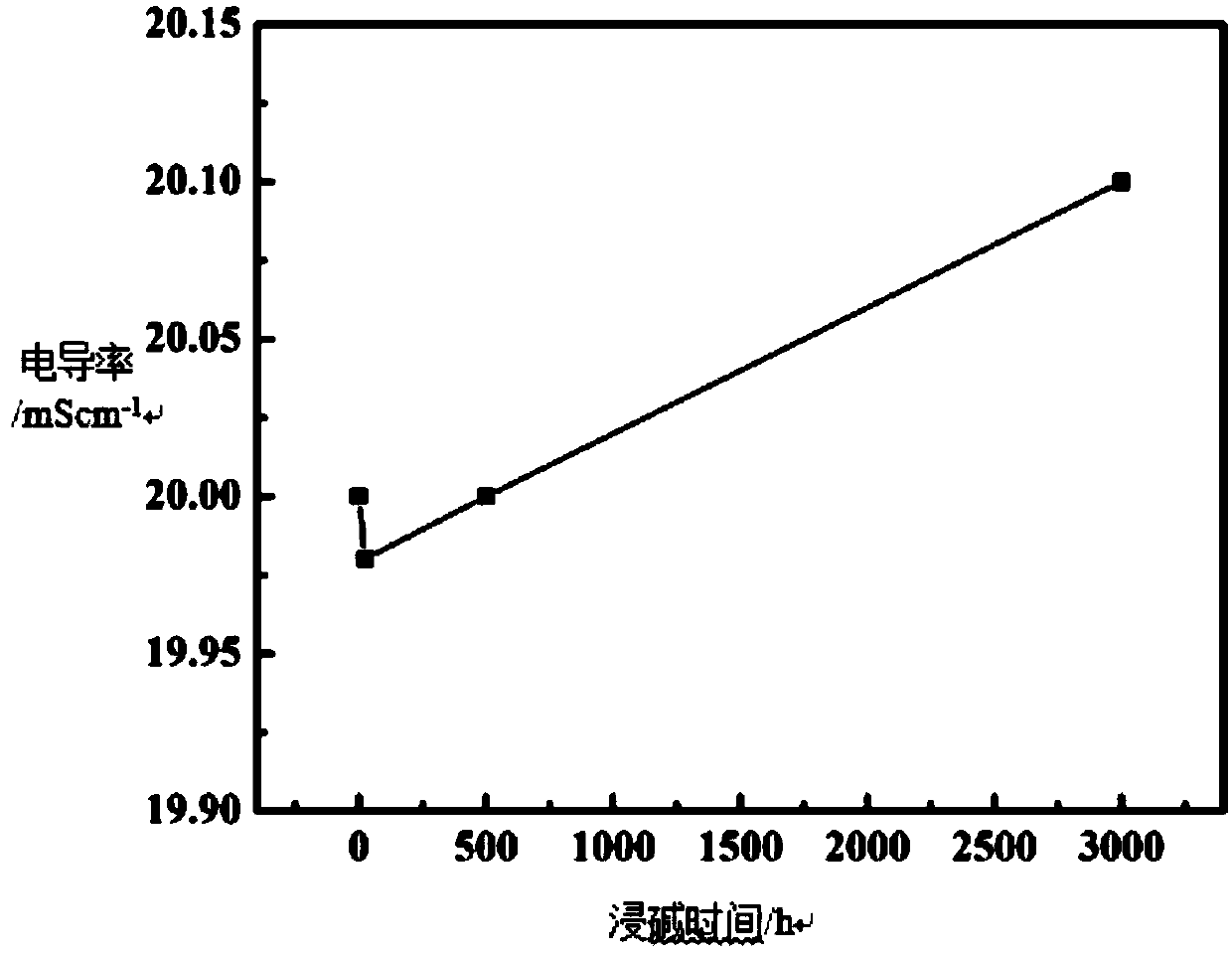
Preparation method of organic-MOF composite alkaline polymer electrolyte membrane and membrane
A technology of electrolyte membranes and polymers, applied in circuits, fuel cells, electrical components, etc., can solve the problems of performance degradation of alkaline membranes, and achieve the effects of optimizing the ratio of chloromethylation and good chemical stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
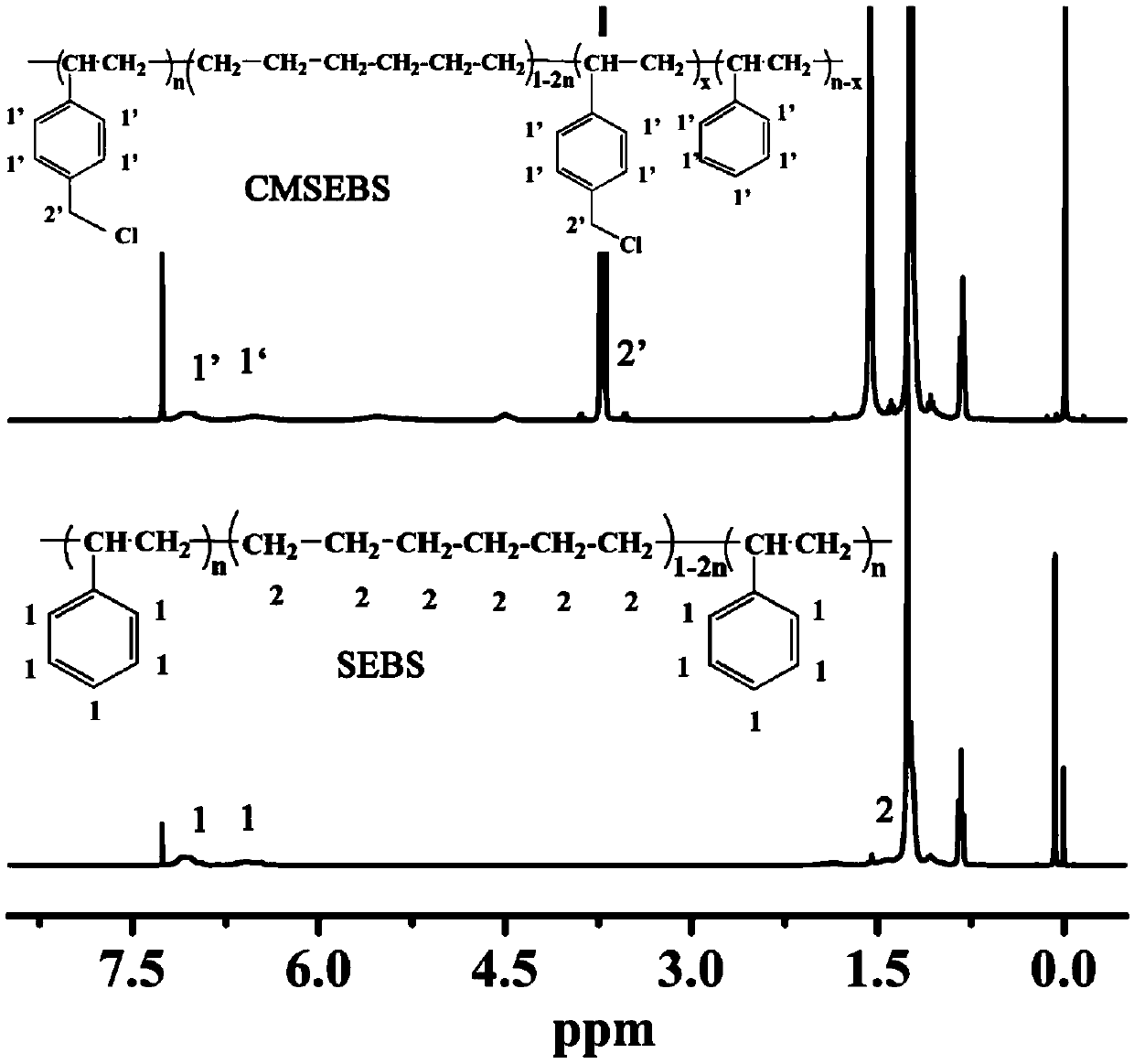
[0036] Dissolve 4g SEBS in 100mL carbon tetrachloride, add 2mL SnCl 4 And 5mL1,4-dichloromethoxybutane, then warmed up to 60°C and maintained at this temperature with magnetic stirring for 8h. After the reaction solution returned to room temperature, it was poured into ethanol to precipitate a yellow solid, which was dissolved in tetrahydrofuran and then precipitated with ethanol. This process was repeated three times, and then fully washed with ethanol, and then the solid was vacuum-dried at room temperature for 8 hours to obtain CMSEBS.
[0037] Dissolve 1.5 g of the above-prepared CMSEBS in 20 mL of chloroform, then add 40 mL of tetrachloroethane, and stir at room temperature to obtain an orange-yellow transparent solution. At the same time, 0.3g ZIF-11 molecular sieve was ultrasonically dispersed in 30mL tetrachloroethane. Then the ZIF-11 / tetrachloroethane dispersion solution was slowly added dropwise to the solution of CMSEBS while stirring, and after the two were fully ...
Embodiment 2
[0050] Dissolve 4g of SEBS in 100mL of carbon tetrachloride, add in sequence in ice-water bath (7°C), 4mL of SnCl 4 And 8mL1,4-dichloromethoxybutane, then warmed up to 60°C and maintained at this temperature with magnetic stirring for 8h. After the reaction solution returned to room temperature, it was poured into ethanol to precipitate a yellow solid, which was dissolved in tetrahydrofuran and then precipitated with ethanol. This process was repeated three times, and then fully washed with ethanol, and then the solid was vacuum-dried at room temperature for 8 hours to obtain CMSEBS, its chlorine The degree of methylation was 0.35.
[0051] Dissolve 0.5 g of the above-prepared CMSEBS in 3 mL of chloroform, then add 15 mL of tetrachloroethane, and stir at room temperature to obtain an orange-yellow transparent solution. At the same time, 0.5g ZIF-8 molecular sieve was ultrasonically dispersed in 30mL tetrachloroethane. Then the ZIF-8 / tetrachloroethane dispersion solution was ...
Embodiment 3
[0053] Dissolve 4g of SEBS in 100mL of carbon tetrachloride, add in sequence in ice-water bath (7°C), 4mL of SnCl 4 And 8mL1,4-dichloromethoxybutane, then magnetically stirred at room temperature for 24h. After the reaction solution returned to room temperature, it was poured into ethanol to precipitate a yellow solid, which was dissolved in tetrahydrofuran and then precipitated with ethanol. This process was repeated three times, and then fully washed with ethanol, and then the solid was vacuum-dried at room temperature for 8 hours to obtain CMSEBS, its chlorine The degree of methylation was 0.2.
[0054] Dissolve 0.5 g of the above-prepared CMSEBS in 2 mL of chloroform, then add 8 mL of tetrachloroethane, and stir at room temperature to obtain an orange-yellow transparent solution. At the same time, ultrasonically disperse 0.05 g of ZIF-6 molecular sieve in 1 mL of tetrachloroethane. Then the ZIF-6 / tetrachloroethane dispersion solution was slowly added dropwise to the solu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resonant frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com