Production of flu vaccine in myceliophthora thermophila
A technology for Myceliophthora thermophila and influenza, which is applied in the direction of antisense single-stranded RNA viruses, microorganisms, microorganisms, etc., and can solve problems such as expensive yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
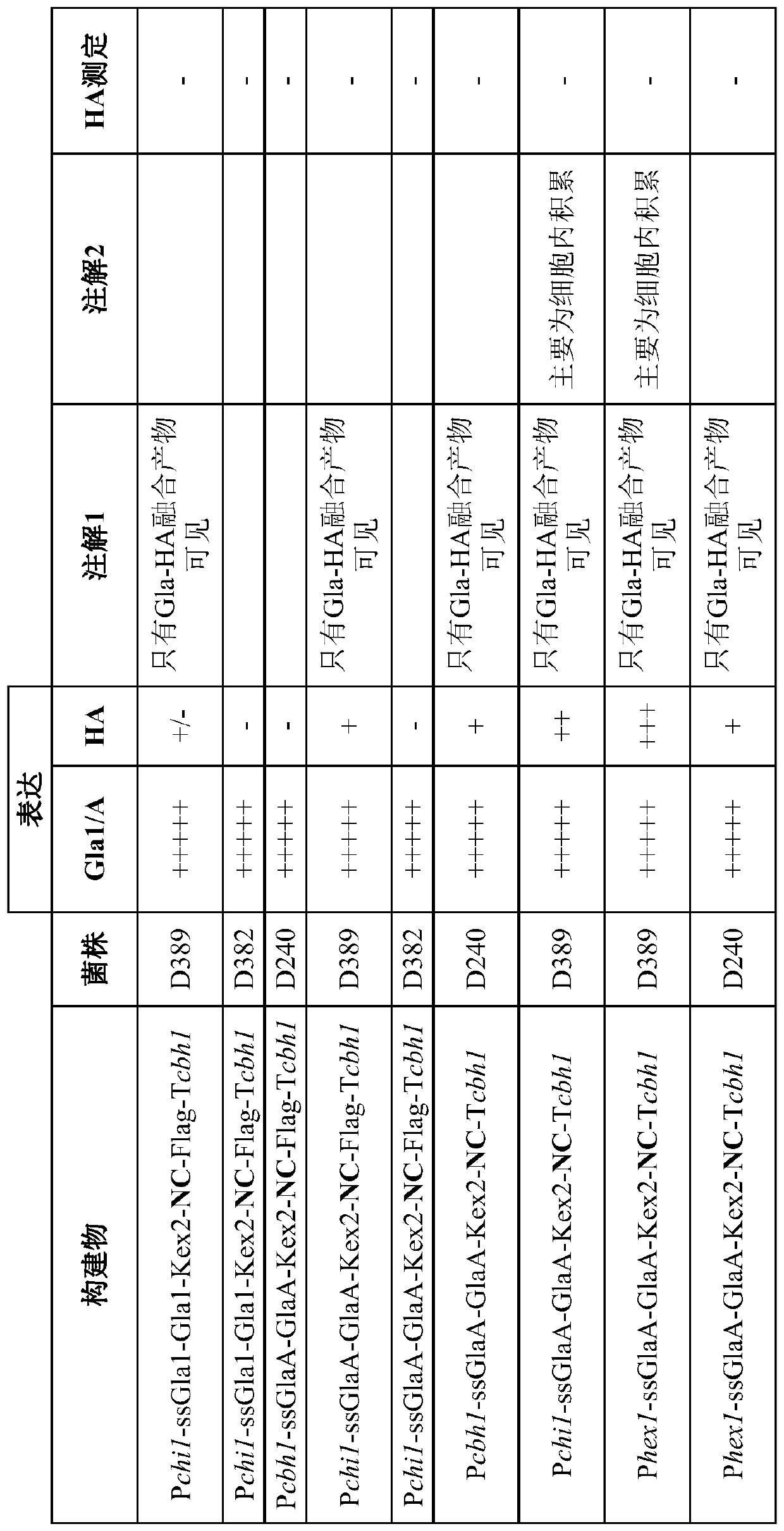
[0122] Example 1 - Hemagglutinin (HA) and neuraminidase (NA) in Myceliophthora (Myceliophthora expression in thermophila) (C1)
[0123] Several series of expression constructs were designed for the expression of recombinant HA proteins of each of the different influenza strains in C1. The HA proteins tested in this study are detailed in Table 1 below. The expression constructs are detailed in Figures 1-4 (Figures 1-3 - Constructs with HA; Figure 4 - Constructs with NA. P = promoter, ss = signal sequence, T = terminator). The type of HA or NA included in each construct is marked in bold. A list of abbreviations used to describe the various elements in the construct is provided at Figure 5 middle. The generation of such constructs is described in detail below under "Materials and Methods".
[0124] Table 1. HA proteins / virus strains
[0125]
[0126]
[0127] Initially, HA was expressed under the cbh1 (cellobiohydrolase 1) or chi1 (chitinase 1) promoter and ...
Embodiment 2
[0227] Example 2 - Immunization of HA produced in Myceliophthora thermophila (C1 ) Originality
[0228] Animal experiments were performed to test the full-length recombinant hemagglutinin protein with a transmembrane domain (rHA-TMD) from the A / New Caledonia / 20 / 99 (H1N1) influenza strain produced in C1 immunogenicity. The biochemical evaluation of rHA-TMD is shown in Figure 9 , which shows analysis of SDS-PAGE, native PAGE, and oligomer status using TEM negative staining. The magnification in the TEM images is x29000. Scale bar = 200 nm. White arrows point to ~40 nm oligomers. White circles mark ~15 nm oligomers.
[0229] Previous immunogenicity studies were performed using a secreted form of rHA from the same strain produced in C1. The transmembrane domain of this rHA is truncated and it contains additional domains (8-Gly domain and T4 foldon domain) that are not present in native HA. The rHA-8Gly-T4 construct did not induce a functional antibody response. It was...
Embodiment 3
[0298] Example 3 - Stirred Tank Fermentation of HA
[0299] Experiments were carried out to measure the production levels of the aforementioned rHA-TMD on a larger scale in stirred tank fermenters using batch and fed-batch techniques. For this, use Minifors TM 3L bioreactor to grow C1 strain D389 expressing rHA-TMD.
[0300] Table 6 summarizes the fermentation conditions for an initial set of experiments performed for 50 hrs. "Batch" - the indicated sugar concentration at the beginning of the fermentation. "Fed-batch" - the concentration of sugar in the feed. Fermentations were performed at pH 7.5 using a starting volume of 1.5 liters.
[0301] Table 6. Fermentation conditions 50hrs
[0302] run number temperature(℃) in batches fed batch R465F2 25 4% xylose none R465F4 35 1% xylose 47% xylose. R465F5 35 1% glucose 50% glucose. R465F6 35 4.7% glucose none
[0303] After fermentation, the mycelium was collecte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com