Refining method of high-purity cariprazine
A refining method and cariprazine technology, applied in the refining field of high-purity cariprazine, can solve problems such as by-product X being difficult to remove
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Cariprazine crude product 10g is joined in the 250ml reaction bottle, add 50ml dichloromethane, 30ml ethanol, heat to reflux, make the solution clarification;
[0038] (2) Slowly add 75ml of ethyl acetate and stir for 20 minutes; slowly cool down to 30°C.
[0039] (3) Ice-water bath, cooled to 0-10°C, stirred for 3 hours;
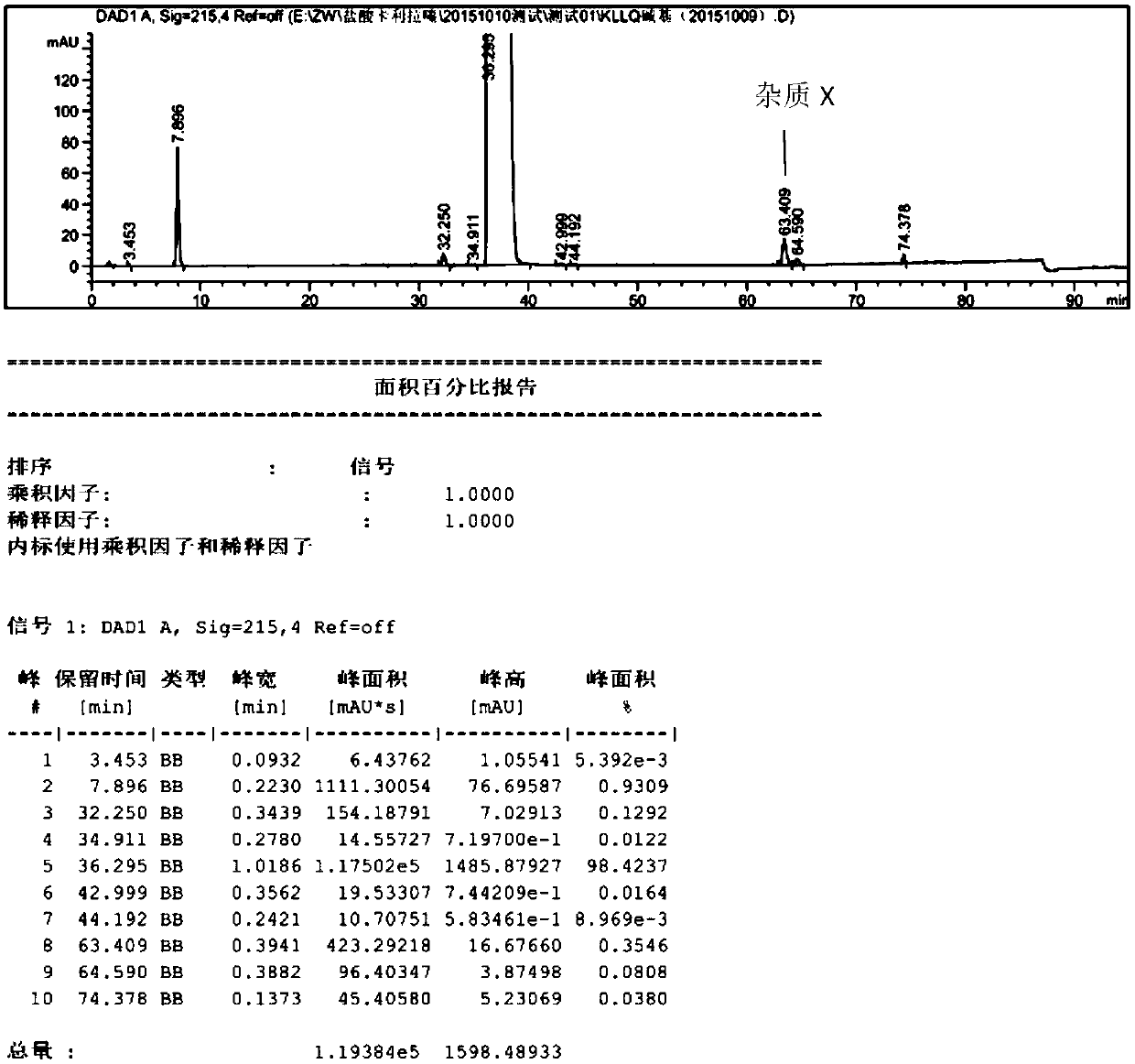
[0040] (4) Filter, and wash the filter cake with 30ml ethyl acetate, and vacuum-dry to constant weight at 50°C to obtain 8.1g cariprazine crystals, yield 81%, purity (HPLC) 99.79, impurity X (63.78min) The content of 0.02%, such as figure 2 mentioned.
Embodiment 2
[0042] (1) Cariprazine crude product 10g is joined in 250ml reaction bottle, add 30ml dichloromethane, 70ml ethanol, heat to reflux, make solution clarification;
[0043] (2) Slowly add 75ml of ethyl acetate and stir for 20 minutes; slowly cool down to 30°C.
[0044] (3) Ice-water bath, cooled to 0-10°C, stirred for 5 hours;
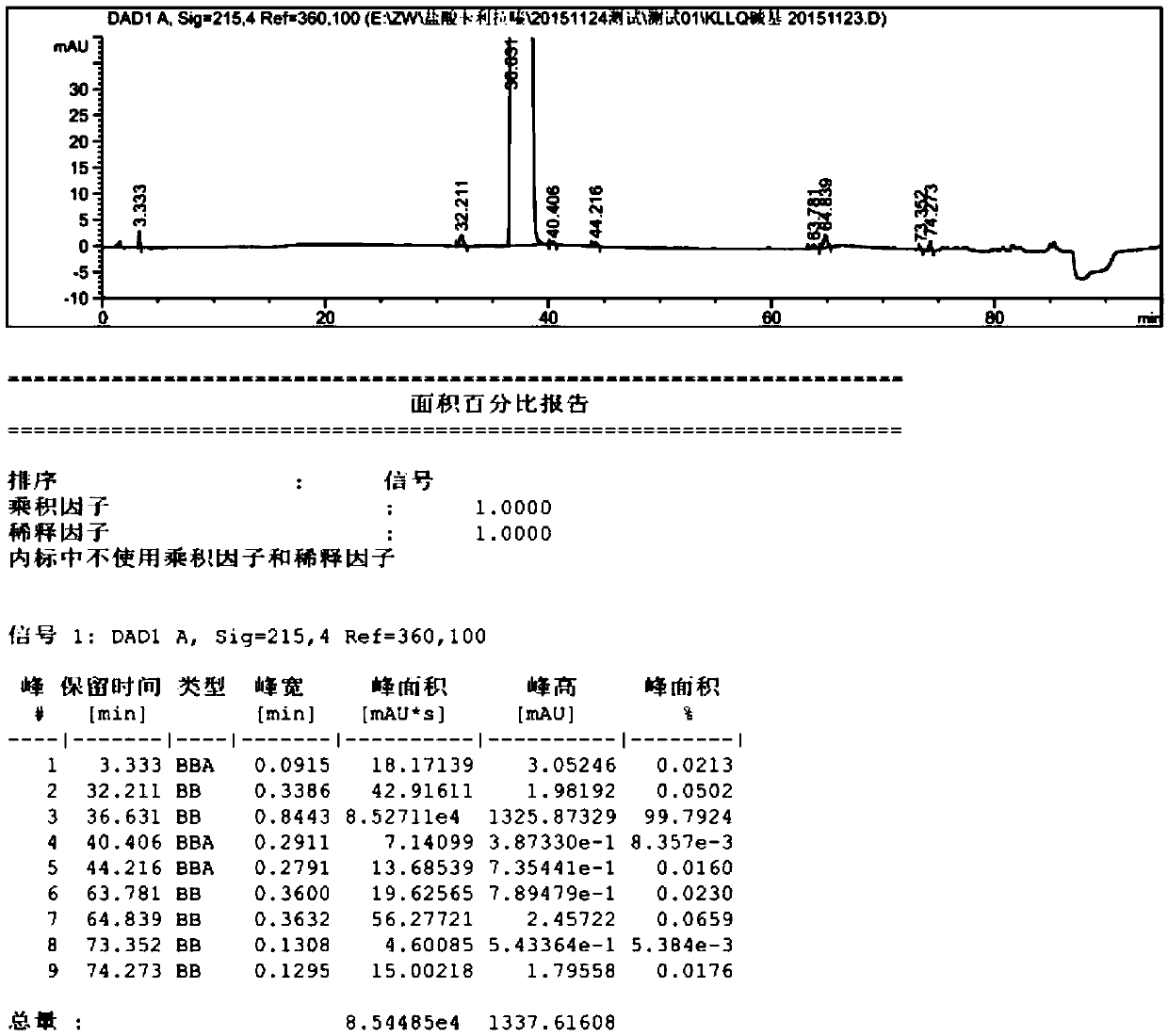
[0045] (4) filter, and wash the filter cake with 15ml ethyl acetate, and vacuum-dry to constant weight at 50 ℃, obtain 8.4g cariprazine crystal, yield 84%, purity (HPLC) 99.68, impurity X (63.33min) The content of 0.04%, such as image 3 shown.
Embodiment 3
[0047] (1) Add 20 g of cariprazine crude product into a 500 ml reaction flask, add 40 ml of methylene chloride and 60 ml of ethanol, and heat to reflux to make the solution clear;
[0048] (2) Slowly add 100ml of ethyl acetate and stir for 20 minutes; slowly cool down to 30°C.
[0049] (3) Ice-water bath, cooled to 0-10°C, stirred for 3 hours;
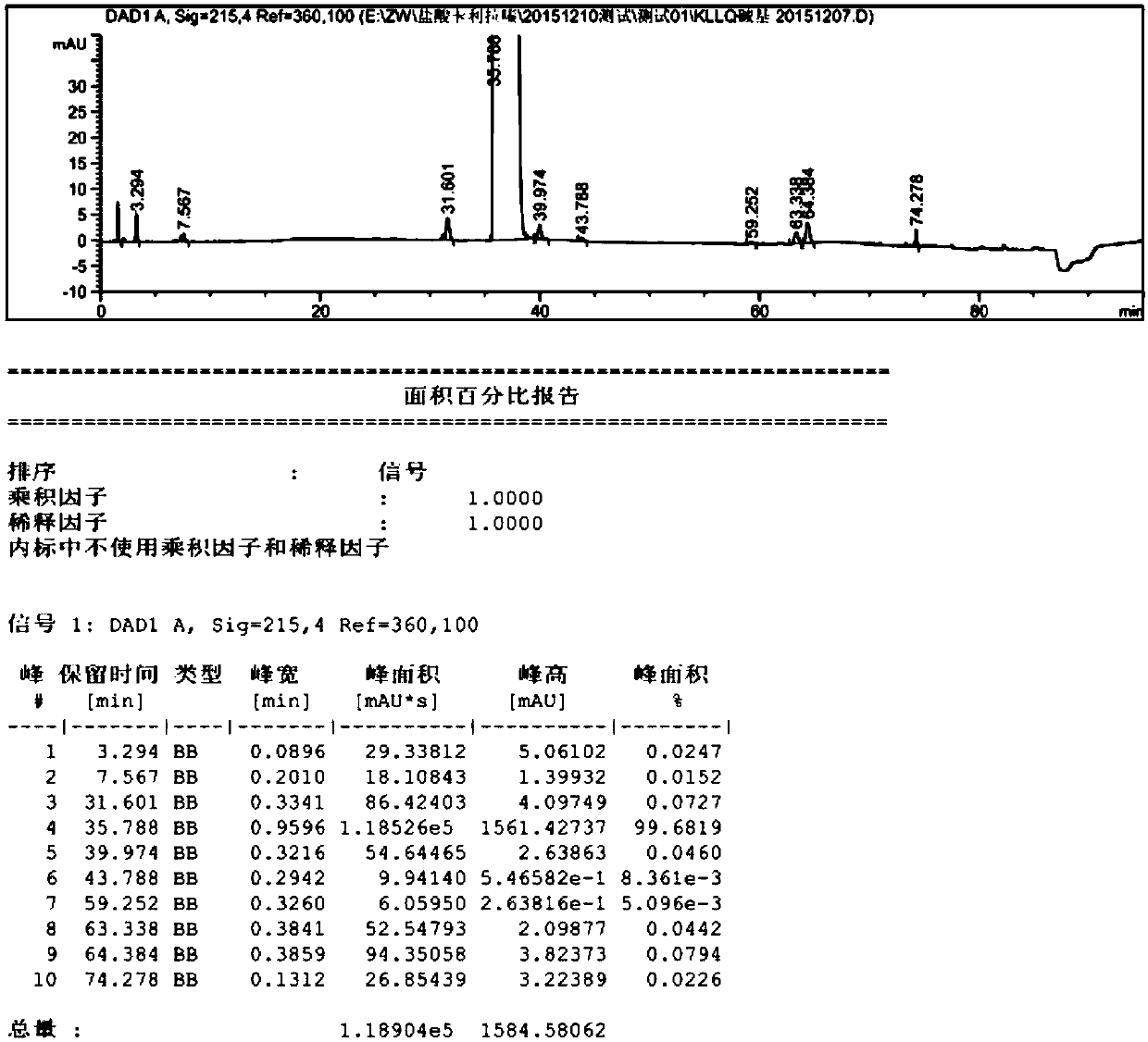
[0050] (4) filter, and wash the filter cake with 30ml ethyl acetate, and vacuum-dry to constant weight at 50 ℃, obtain 17.1g cariprazine crystal, yield 85.5%, purity (HPLC) 99.88, the content of impurity X 0.02% ,Such as Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com