Selective synthesis method of 2-chloro-5-methylpyridine and 2, 3-dichloro-5-methylpyridine
A technology of methyl pyridine and a synthetic method, applied in the direction of organic chemistry and the like, can solve the problems of only 56% of the total reaction yield, only 60% of the conversion rate, slow research and development progress, etc., and achieves low production cost, easy operation, and equipment investment. less effect
- Summary
- Abstract
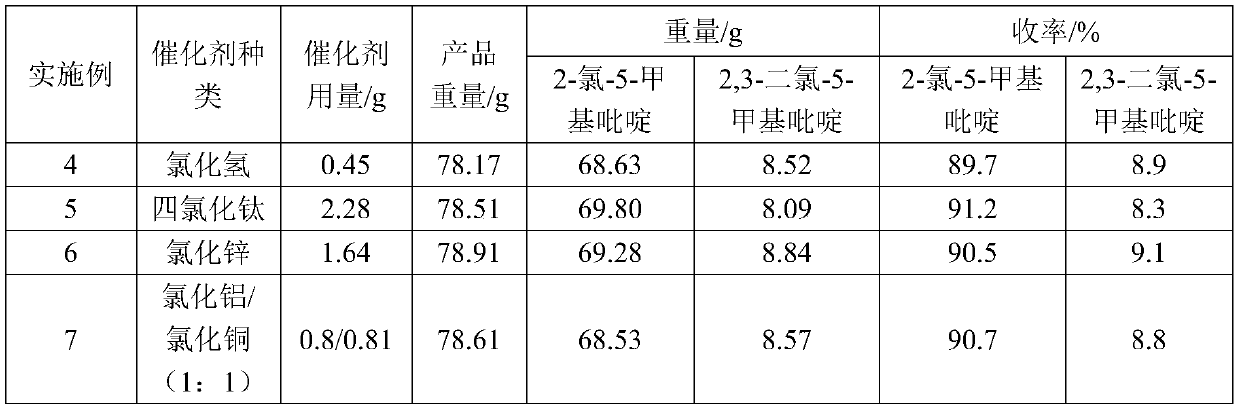
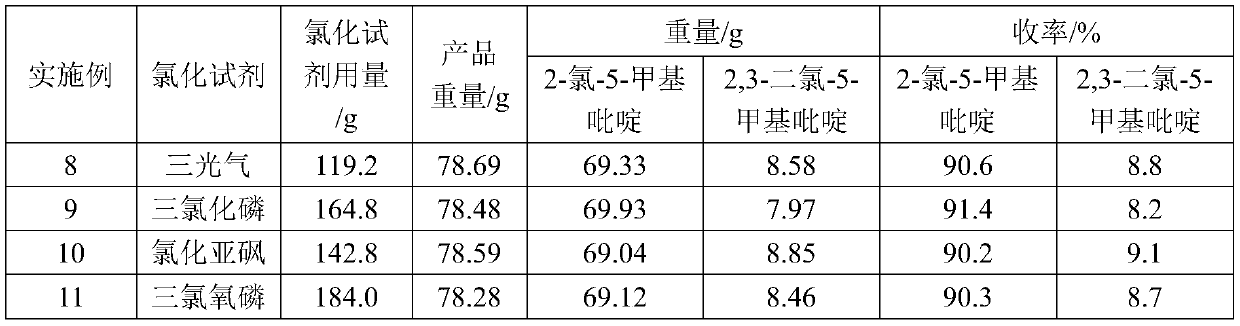
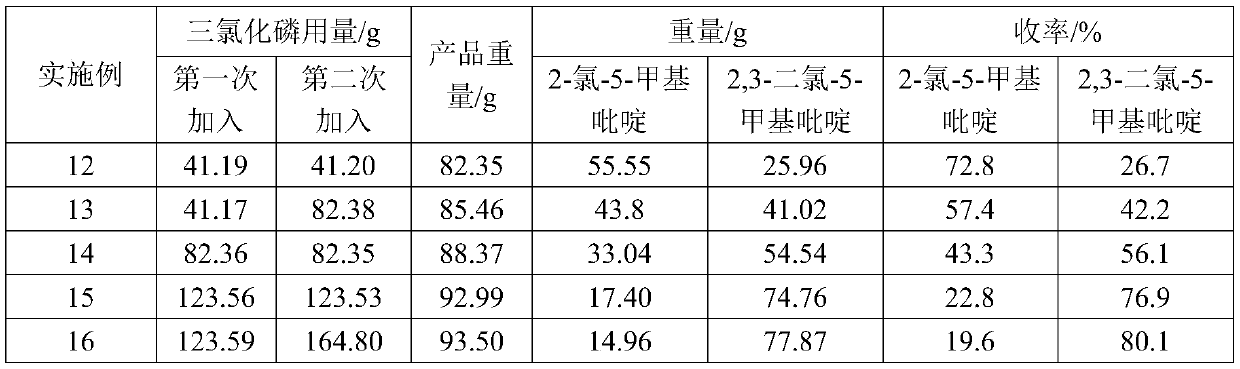
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] At 20°C, add 66.7g (0.6mol) of pyridone, 333.5g of toluene, and 2.02g (0.012mol) of ferric chloride into a 500ml four-necked flask, and mix well. Then feed chlorine gas (5.5L / h) in the mixed solution for 3 hours, the reaction is completed, be warming up to 110°C, feed phosgene (9L / h) for 3 hours, react for 1 hour, and obtain product 78.52g through post-processing steps, Among them, 70.11g of 2-chloro-5-methylpyridine and 7.87g of 2,3-dichloro-5-methylpyridine. The yield of 2-chloro-5-methylpyridine to pyridone was 91.6%, and the yield of 2,3-dichloro-5-methylpyridine to pyridone was 8.1%.
Embodiment 2
[0021] At 20°C, add 66.7g (0.6mol) of pyridone, 333.5g of toluene, 9.8g (0.06mol) of ferric chloride into a 500ml four-necked flask, and feed phosgene (9L / h) for 3 hours; then Chlorine gas (9.5L / h) was passed into the mixed solution simultaneously for 3h, the reaction was completed, the temperature was raised to 110°C, phosgene (9L / h) was passed through again for 3 hours, and the reaction was carried out for 1 hour, and 93.95g of the product was obtained through the post-processing step. Among them, 14.16g of 2-chloro-5-methylpyridine and 79.03g of 2,3-dichloro-5-methylpyridine. The yield of 2-chloro-5-methylpyridine to pyridone was 18.5%, and the yield of 2,3-dichloro-5-methylpyridine to pyridone was 81.3%.
Embodiment 3
[0023] Change ferric trichloride, phosgene and chlorine consumption on the basis of embodiment 2, add ferric trichloride 4.87g (0.03mol), pass into phosgene (4.5L / h) 3 hours, pass into chlorine gas (7.5L / h) for 3 hours. After the reaction, 88.43 g of the product was obtained through post-processing steps, including 33.07 g of 2-chloro-5-picoline and 54.92 g of 2,3-dichloro-5-picoline. The yield of 2-chloro-5-methylpyridine to pyridone was 43.2%, and the yield of 2,3-dichloro-5-methylpyridine to pyridone was 56.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com