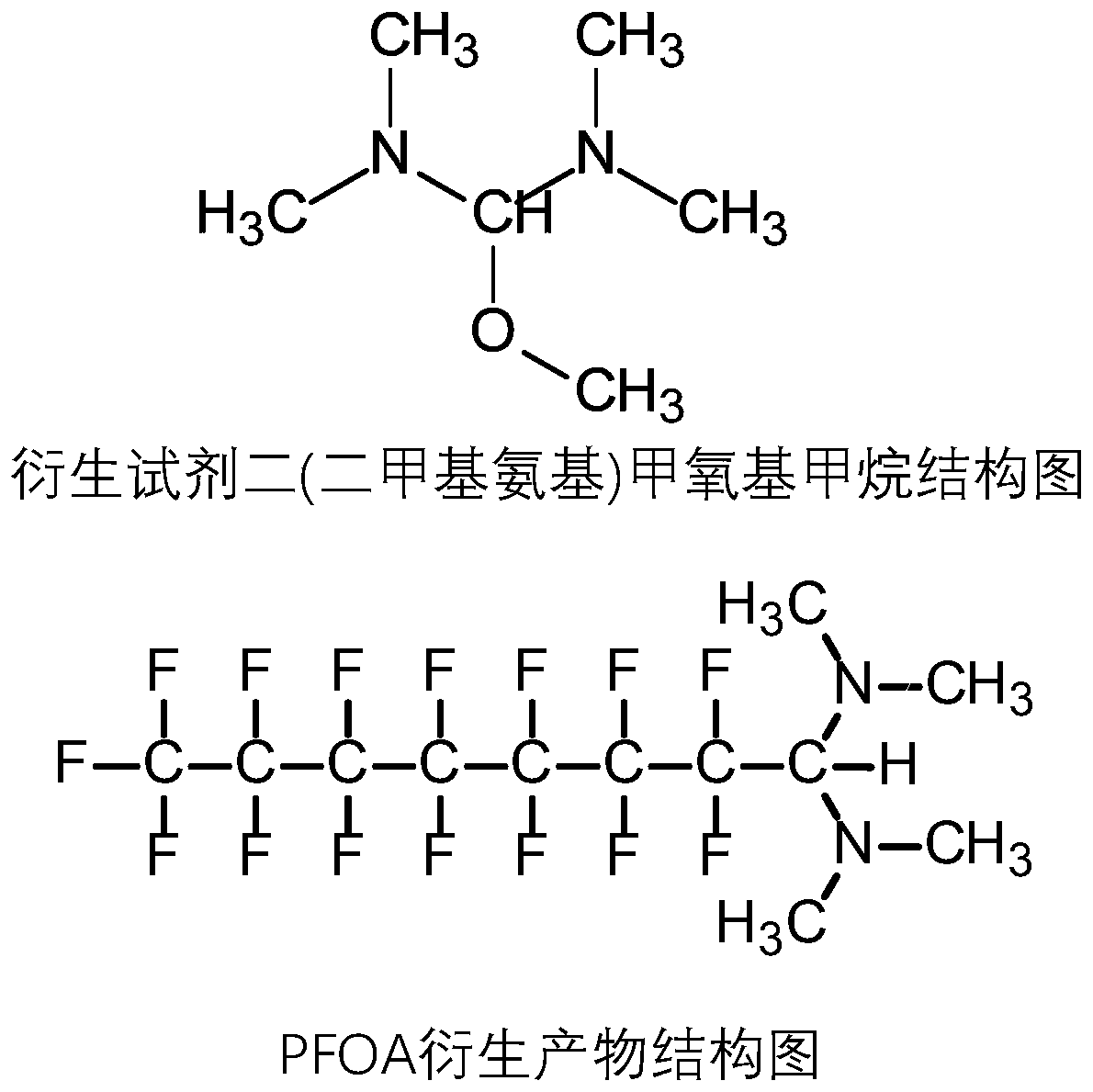
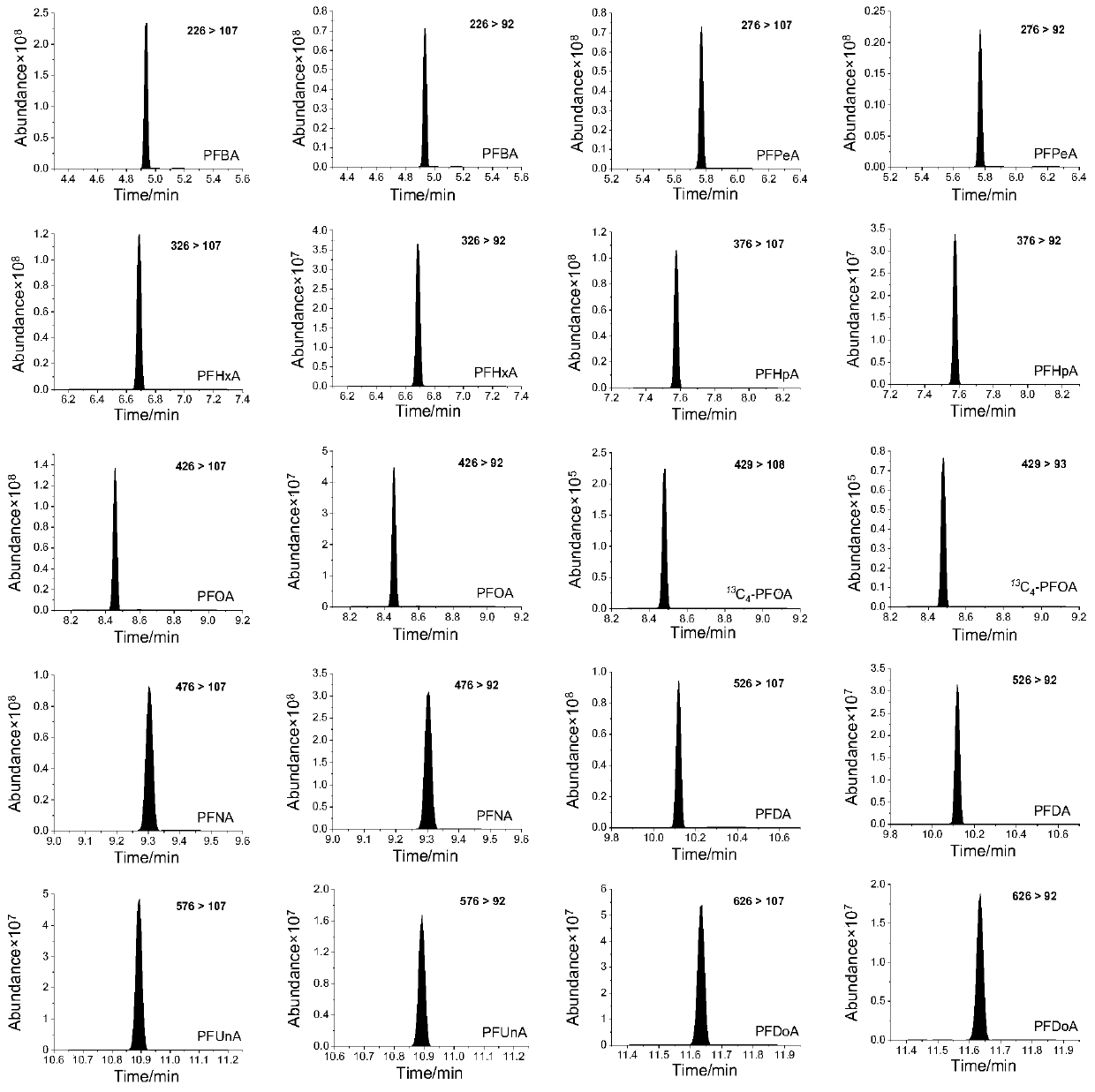
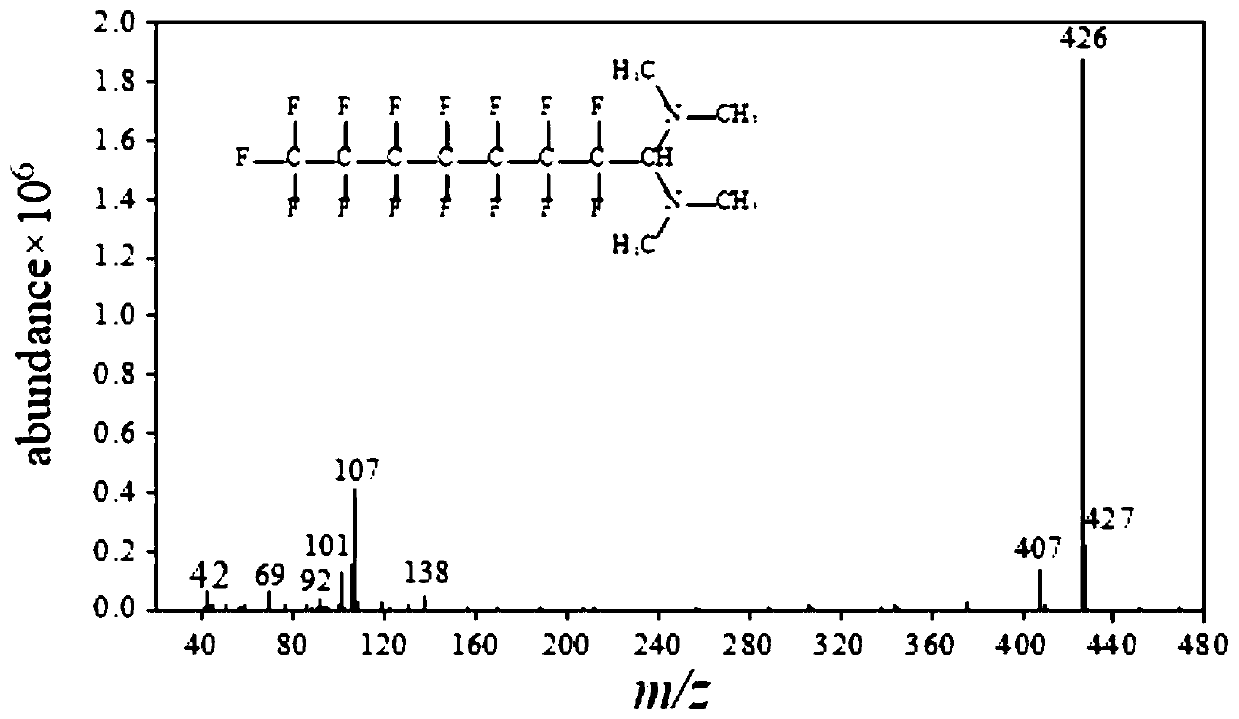
Detection method of perfluorocarboxylic acid compound
A technology of perfluorocarboxylic acid and detection method, applied in the field of chemical detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Quantitative Standard Curve Drawing, Method Detection Limit and Precision
[0057] (1) Take multiple parts of ethyl acetate solution, add appropriate amount of perfluorocarboxylic acid compound standard substance and internal standard therein, mix well,
[0058] (2) The concentration range of each perfluorocarboxylic acid standard substance in the standard curve is set as: 0.5-500ng / mL;
[0059] (3) Set 10 concentrations of the target substance (0.5, 1, 2, 5, 10, 20, 50, 100, 200, 500 ng / mL respectively); the concentration of the internal standard is 100 ng / mL.
[0060] (4) Add 20 μL of bis(dimethylamino)methoxymethane, and derivatize in an oven at 35° C. for 30 minutes.
[0061] (5) Perform instrumental testing according to the method of the present invention.
[0062] Take the concentration X of perfluorocarboxylic acid as the abscissa, and take the ratio Y of the peak area of the perfluorocarboxylic acid derivative to the peak area of the internal sta...
Embodiment 2
[0074] The optimization of embodiment 2 derivatizing agent adding amount
[0075] (1) Take 10 μL of 500 mg / L perfluorocarboxylic acid mixed standard solution and add it to the derivation bottle;
[0076] (2) Add 1 μL, 2 μL, 5 μL, 10 μL, 20 μL, and 50 μL of bis(dimethylamino)methoxymethane respectively, and dilute to 1 mL with ethyl acetate;
[0077] (3) React at 35° C. for 30 minutes, cool down, and transfer the solution after passing through a 0.22 μm organic filter membrane to an injection bottle for gas chromatography-tandem mass spectrometry (GC-MS / MS) detection.
[0078] The experimental results show that as the amount of derivatizer increases, the peak area of perfluorocarboxylic acid derivatives gradually increases, and a larger peak area can be obtained in the range of 5-50 μL, of which 20 μL is the optimal value of the amount of derivatizer .
Embodiment 3
[0079] The optimization of embodiment 3 derivation time
[0080] (1) Take 10 μL of 500 mg / L perfluorocarboxylic acid mixed standard solution and add it to the derivation bottle;
[0081] (2) Add 20 μL of bis(dimethylamino)methoxymethane, and dilute to 1 mL with ethyl acetate;
[0082] (3) React at 35°C for 5, 10, 30, 60, 120, and 240 min respectively, cool down, and transfer the solution after passing through a 0.22 μm organic filter membrane to an injection bottle for gas chromatography-tandem mass spectrometry (GC- MS / MS) detection.
[0083] The experimental results show that the larger peak area can be obtained when the reaction time is within 5-240 minutes, and 30 minutes is the optimal time for the derivatization reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com