Indole-based heterocyclic compound as well as preparation method and application thereof in prevention and treatment of plant diseases
A kind of heterocyclic compound and compound technology, which is applied to indole heterocyclic compound and its preparation and application field in preventing and controlling plant diseases, and achieves the effect of good anti-plant virus and pathogenic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
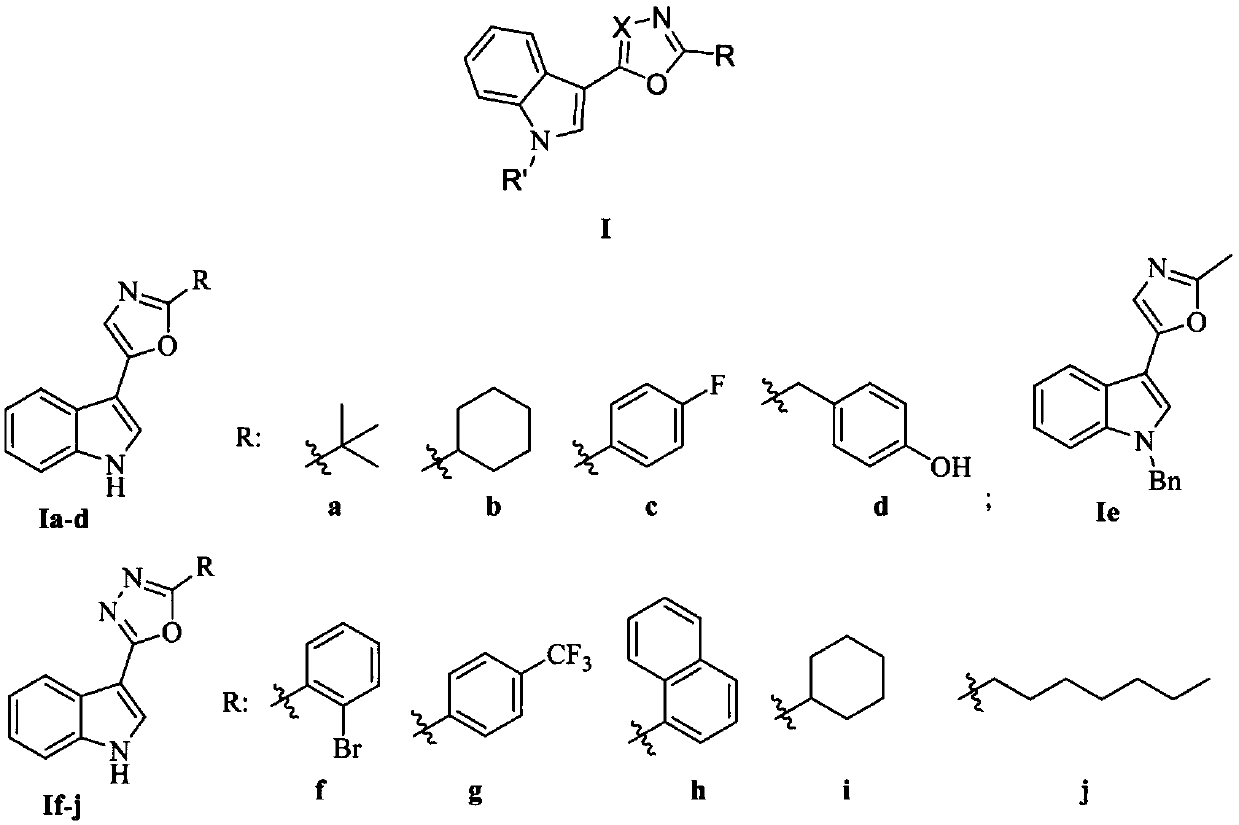
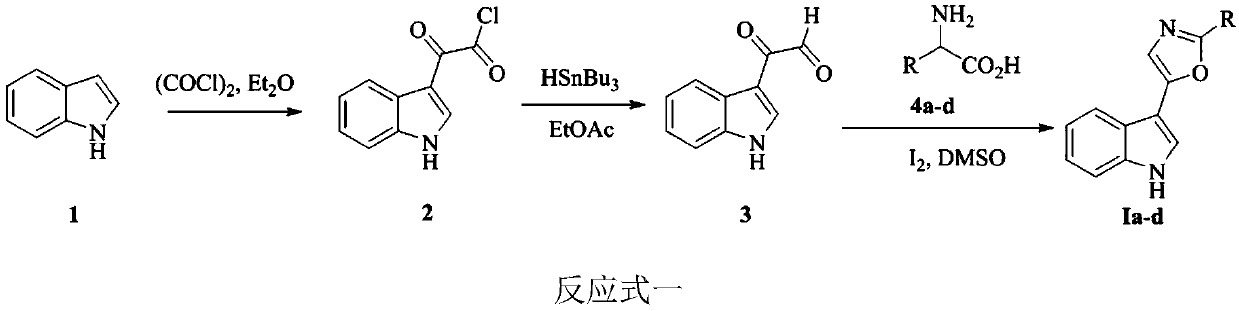
[0049] Embodiment 1: the synthesis of indole heterocyclic compound Ia-d
[0050]
[0051] Indole carbonyl acetyl chloride: Weigh 20g of indole and add it to a 1L four-neck flask, add 350mL of anhydrous ether under mechanical stirring, after the solid is completely dissolved, slowly add 17.3mL of oxalyl chloride dropwise at 0°C, and it takes 30 minutes to complete the addition . The reaction solution was reacted at 0° C. for 3 h, then raised to room temperature and then reacted for 1 h. After the reaction was completed, it was filtered with suction and washed three times with anhydrous ether. After drying, 32 g of compound 2 (ie, indolecarbonyl acetyl chloride) was obtained, with a yield of 90%, which could be directly used in the next reaction after being characterized.
[0052] Indolecarbonylacetaldehyde 3: Add the above-prepared product 2 into a 500mL four-necked round-bottomed flask, then add 153mL of ethyl acetate, and add 42.9mL of tri-n-butyltin hydride dropwise in ...
Embodiment 2
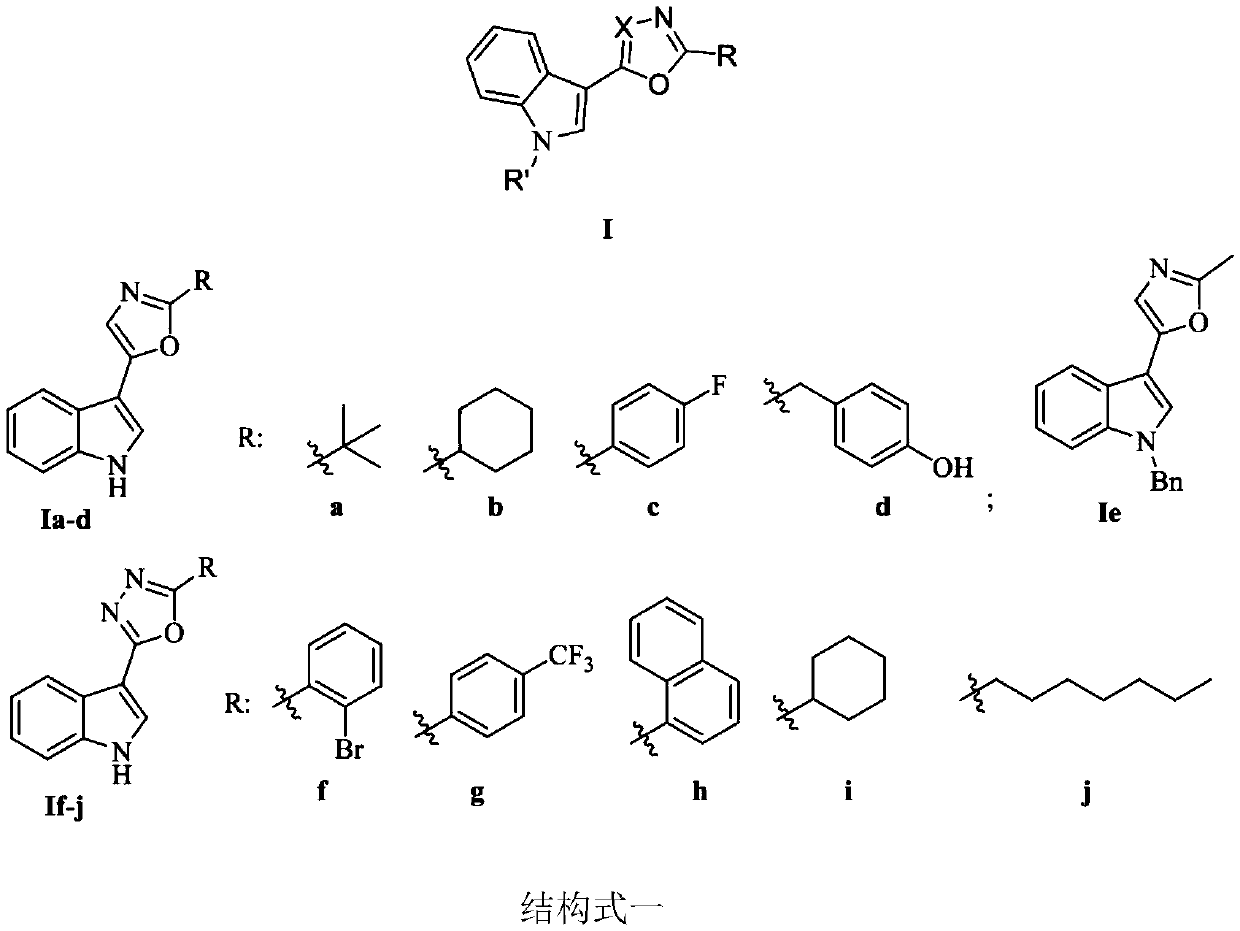
[0058] Embodiment 2: the synthesis of indole heterocyclic compound Ie
[0059]
[0060] Indole heterocyclic compound 5: In a 100mL four-neck round bottom flask, add indole carboaldehyde (5.8mmol), alanine amino acid 4e (11.6mmol), 30mL dimethyl sulfoxide solvent, solid iodine (5.8mmol) , the mixture was mechanically stirred and reacted for 30min under a nitrogen atmosphere at 110°C. After the reaction was completed, a saturated sodium thiosulfate solution (20mL) was added to react for 10min, extracted, dried over anhydrous magnesium sulfate, then suction filtered, and the product compound was obtained by column chromatography 5. Brown solid, melting point 202-203°C, yield 66%; 1 HNMR (400MHz, DMSO-d 6 )δ11.54(s,1H,NH),7.84(d,J=7.9Hz,1H,Ar-H),7.74(d,J=2.6Hz,1H,Ar-H),7.47(d,J= 8.0Hz,1H,Ar-H),7.30(s,1H,Ar-H),7.12–7.23(m,2H,Ar-H),2.49(s,3H,CH 3 ); 13 C NMR (100MHz, DMSO-d 6 )δ158.2, 147.3, 136.3, 123.5, 122.8, 122.0, 120.0, 119.4, 119.2, 112.0, 103.9, 13.6; HRMS (ESI) cal...
Embodiment 3
[0062] Embodiment 3: the synthesis of indole heterocyclic compound If-j
[0063]
[0064]Indolehydrazone 8a-e: In a 100mL round bottom flask equipped with a magnetic stirrer, add indole-3-carboxylhydrazide (6mmol), the corresponding aldehyde (6mmol), absolute ethanol (80mL), and the mixture was dissolved at 80 After reacting at ℃ for 1 hour, cool naturally to room temperature, and use a rotary evaporator to evaporate until 15-20 mL of liquid remains, and then stand still and filter with suction to obtain acylhydrazones 8a-e. After nuclear magnetic detection, the data are as follows.
[0065] 8a. White solid, melting point 294-296°C, yield 92%; 1 H NMR (400MHz, DMSO-d 6 )δ11.81(s,1H,NH),11.71(s,1H,NH),8.68(s,1H,Ar-H),8.30(s,1H,Ar-CH),8.23(d,J=7.3 Hz,1H,Ar-H),8.02(dd,J=6.6,1.2Hz,1H,Ar-H),7.70(d,J=8.0Hz,1H,Ar-H),7.46–7.51(m,2H ,Ar-H),7.33–7.37(m,1H,Ar-H),7.16–7.24(m,2H,Ar-H); 13 C NMR (100MHz, DMSO-d 6 )δ136.0, 133.5, 130.1, 131.2, 128.0, 127.0, 123.1, 122.3, 120.9, 112....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com