Sonodynamic therapy
A chemotherapeutic agent and sound sensitizer technology, applied in the field of improvement of sonodynamic therapy, can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0206] Example 1 - Synthesis of Biotin-Gemcitabine (Biotin-Gem) and Biotin-Gemcitabine-Rose Bengal (Biotin-Gem-RB) Conjugates
[0207] 1.1 Synthesis of biotinylated gemcitabine (biotin-GEM):
[0208] Biotinylated gemcitabine was synthesized according to Protocol 1. The program is provided below.
[0209]
[0210] Scheme 1: Synthetic scheme for preparing biotin-Gem
[0211] (2R,3R,5R)-5-(4-Amino-2-oxopyrimidin-1(2H)-yl)-4,4-difluoro-3hydroxytetrahydrofuran-2-yl)methyl(2-( Synthesis of 5-(2-oxohexahydro-1H-thieno[3,4-d)imidazol-4-yl)pentanamido)ethyl)carbonate (4):
[0212]Compound 2 was prepared following literature procedures (see McEwan et al. Biomaterials. 2016:80, 20-32). To a solution of 2 (0.28 g, 0.9 mmol) in DCM (10 mL) was added 4-nitrophenylchloroformate (0.59 g, 2.9 mmol), DIPEA (0.50 g, 3.9 mmol) and a catalytic amount of pyridine and stirred at room temperature for 24 hours. The reaction mixture was then concentrated to dryness in vacuo. The crude resid...
Embodiment 2
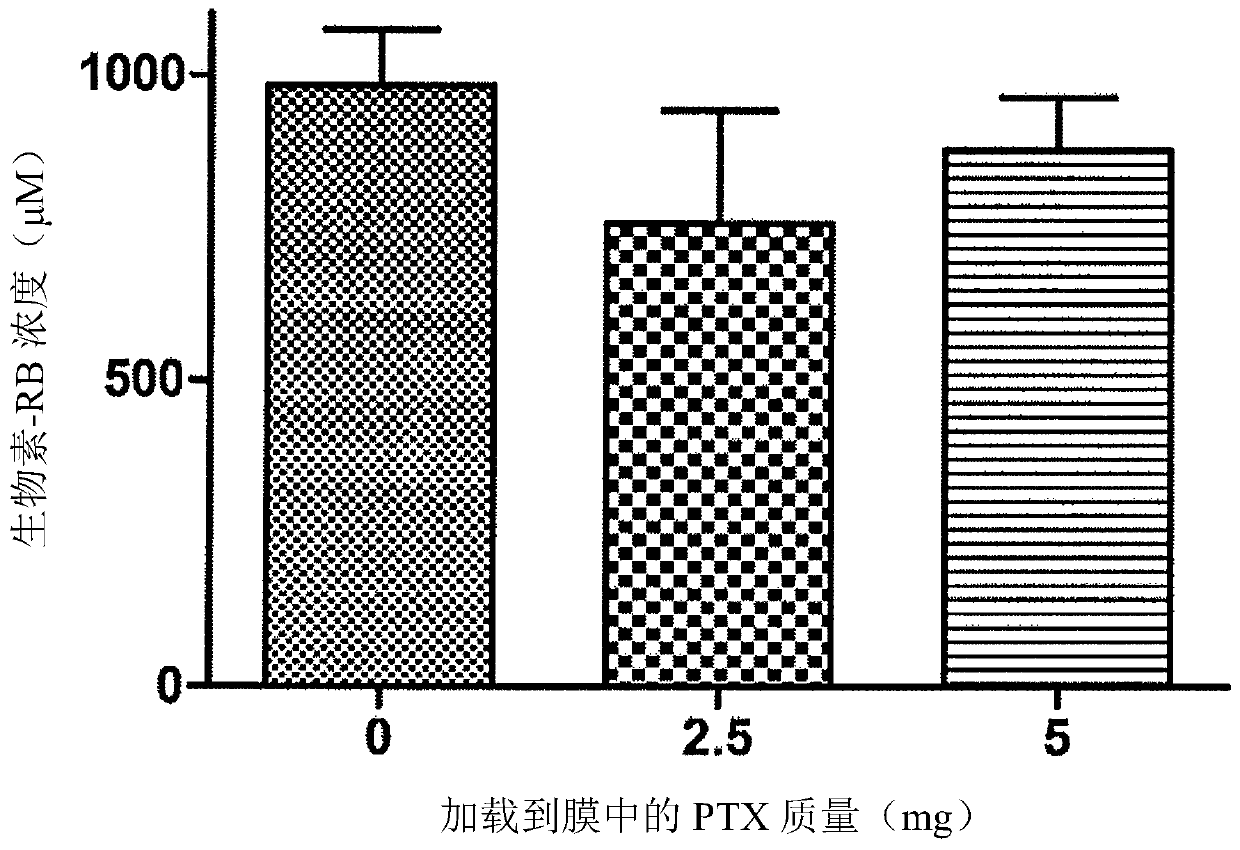
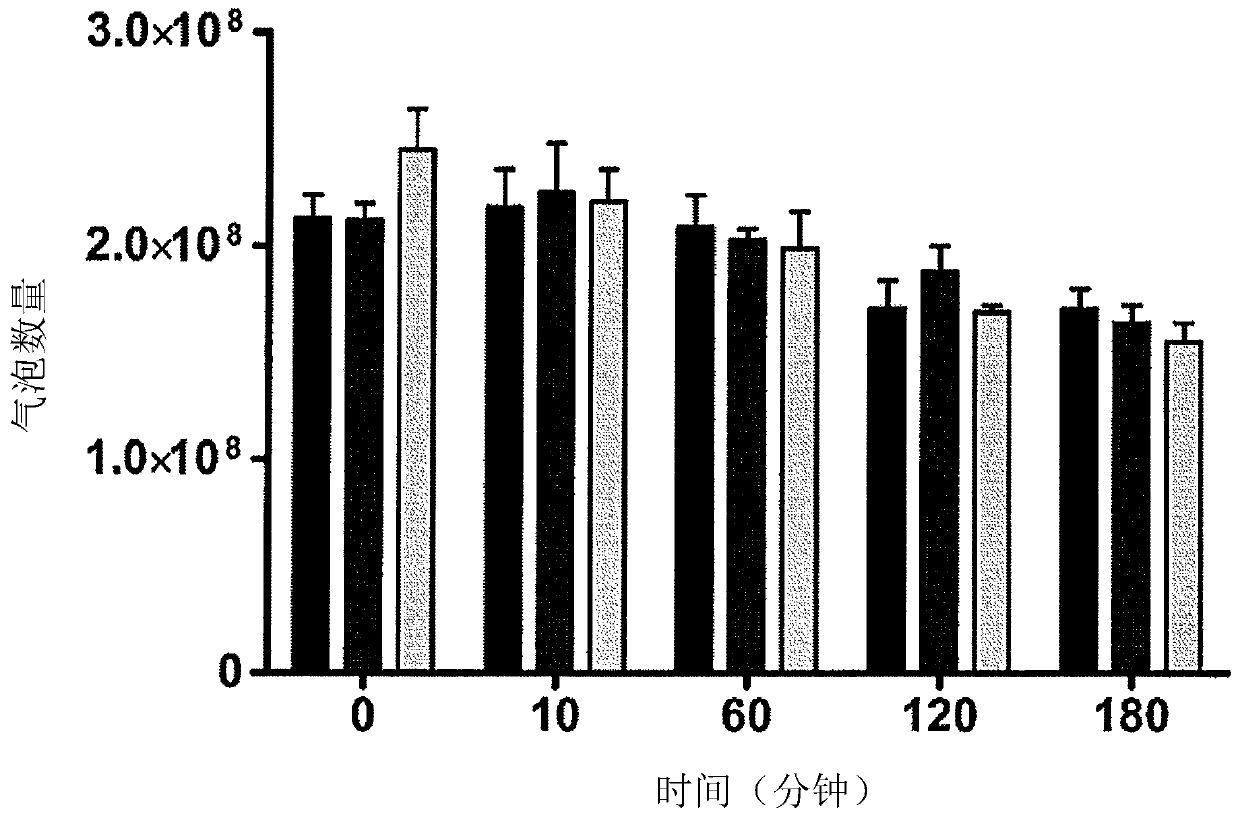
[0232] Example 2- Preparation, Stability and Loading Efficiency of Avidin Functionalized Paclitaxel (PTX) Loaded Microbubbles (MB)
[0233] 2.1 Preparation of lipid-stabilized MB (PTX-MB) doped with PTX in the shell:
[0234] By first adding the lipids 1,2-dibehenyl-sn-glycero-3-phosphocholine (DBPC) (4.0mg, 4.43umol), 1,2-distearoyl-sn-glycero-3-phosphate Ethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG(2000)) (1.35mg, 0.481μmol) and DSPE-PEG(2000)-biotin (1.45mg, 0.481μmol) dissolved Avidin-functionalized lipid-stabilized microbubbles with hydrophobically incorporated PTX in the shell were prepared in chloroform to achieve a molar ratio of 82:9:9. To this solution was added paclitaxel (5 mg, 5.86 μmol) dissolved in chloroform. The solution was heated at 40°C for 30 minutes until the chloroform evaporated. The dried lipid film was reconstituted in 3 mL of Ringer's solution (PBS, glycerol, propylene glycol (8:1:1 by volume)) and heated on a 75 °C water bath for ...
Embodiment 3
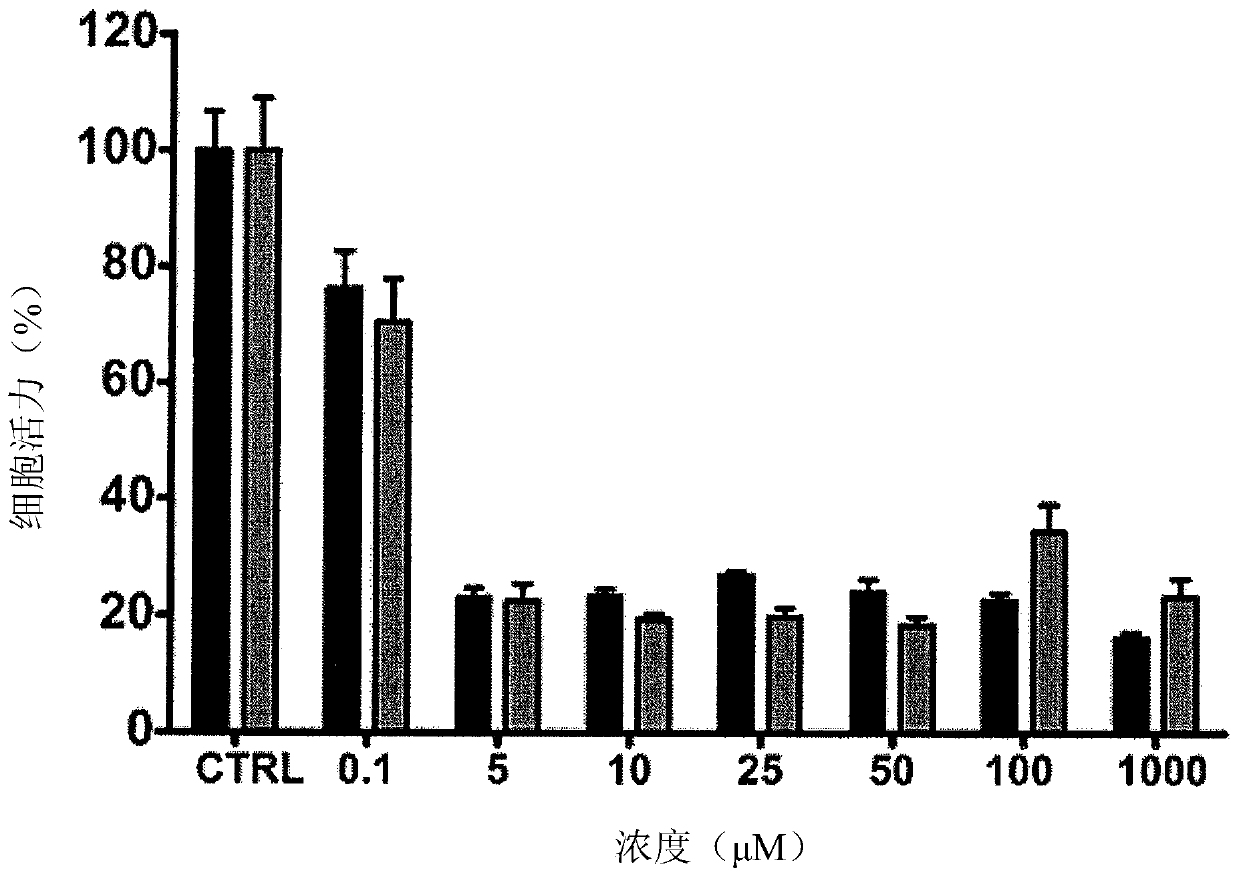
[0243] Example 3 - Biological testing of PTX-MB-RB, PTX-MB-Gem and PTX-MB-Gem-RB
[0244] 3.1 Efficacy of Biotin-Gem and Biotin-Gem-RB in BxPC-3 cells:
[0245] To ensure that the antimetabolite efficacy of biotin-Gem and biotin-Gem-RB was not affected by chemical modification, the cytotoxicity of the conjugates was determined in the human pancreatic cancer cell line BxPc-3. at 37°C in humidified 5% CO 2 Cells were maintained in RPMI-1640 supplemented with 10% (v / v) fetal calf serum in atmosphere. These cells were seeded into 96-well plates at a density of 5000 cells per well. Plates were then incubated for 24 hours before adding 100 uL of media spiked with Gem, Biotin-Gem or Biotin-Gem-RB. The concentrations used for Biotin-Gem were 0, 0.1, 5, 10, 25, 50, 100 and 100 μM, while for Biotin-Gem-RB, 0.01 μM, 0.05 μM, 0.1 μM, 0.5 μM, 1 μM, Concentrations of 5 μM and 10 μM. In each case the corresponding concentration of Gem was used. Cells were then incubated for a further...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com