Herpetetrone nanosuspension and dry powder thereof, and preparation methods and applications of herpetetrone nanosuspension and dry powder
A nano-suspension, dry powder technology, applied in powder delivery, pharmaceutical formulations, medical preparations with non-active ingredients, etc., can solve the problems of affecting drug efficacy, low solubility, slow dissolution rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1: Preparation of Herpetetrone Nanosuspension:
[0040] (1) Accurately weigh 50 mg of the HPT bulk drug, place it in 10 mL of absolute ethanol and dissolve it completely to obtain solution A;
[0041] (2), accurately weigh povidone K30 10mg into 40mL distilled water and dissolve completely to obtain solution B;
[0042] (3), put solution B under the probe for ultrasonication, quickly inject solution A into solution B, and continue ultrasonication for 15 minutes to obtain Herpetetrone nanosuspension; the mode of ultrasonication is working for 3s and intermittent for 3s; The power is 400W.
Embodiment 2
[0043] Example 2: Preparation of Herpetetrone nanosuspension dry powder:
[0044] (1), preparation of Herpetetrone nano-suspension: accurately weigh 50 mg of HPT bulk drug, place in 20 mL of absolute ethanol and dissolve completely to obtain solution A;
[0045] Accurately weigh 3010 mg of PVPK into 40 mL of distilled water and dissolve completely to obtain solution B;
[0046]Put solution B under the probe and sonicate (work for 3s, intermittent for 3s, power 400W); quickly inject solution A into solution B, and continue to sonicate for 15min with the same ultrasonic mode and frequency to obtain Herpetetrone nanosuspension;
[0047] (2) Preparation of Herpetetrone nanosuspension dry powder: put the HPT-NS prepared in step (1) in a refrigerator at -20°C for 24 hours, and then put it in a freeze dryer with a pressure of 0.10mbar and a temperature of -50°C Freeze-dried for 72 h and then taken out to obtain Herpetetrone nanosuspension dry powder.
Embodiment 3
[0048] Example 3: Preparation of Herpetetrone nanosuspension dry powder:
[0049] In this embodiment, the preparation process of Herpetetrone nanosuspension is the same as that of Example 2, the difference being the preparation of Herpetetrone nanosuspension dry powder, which is obtained by spray drying in this embodiment, and the specific steps are: The HPT-NS prepared in step (1) was spray-dried according to the following conditions: inlet air temperature 100°C, exhaust air temperature 60°C, compressed gas flow rate 465L / h, pumping rate 41m 3 / h, and the spray speed of the feed liquid is 3ml / min to obtain the dry powder of Herpetetrone nanosuspension.
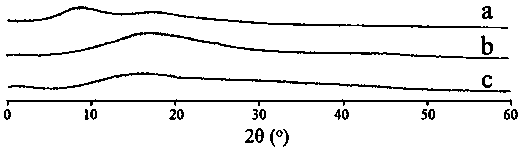
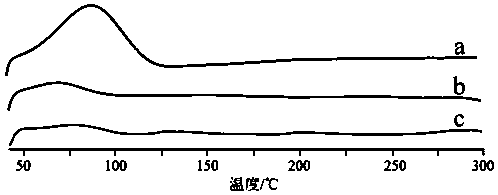
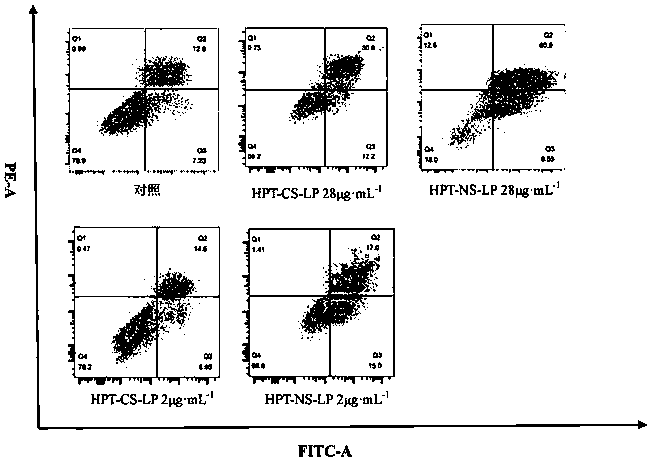
[0050] The determination of a kind of Herpetetrone nanosuspension of the present invention and its dry powder preparation method and its particle size, dispersion index, in vitro dissolution rate, investigation of its inhibitory effect on HSC-T6 liver fibroblasts of the present invention and its comparison with ordinary sus...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com