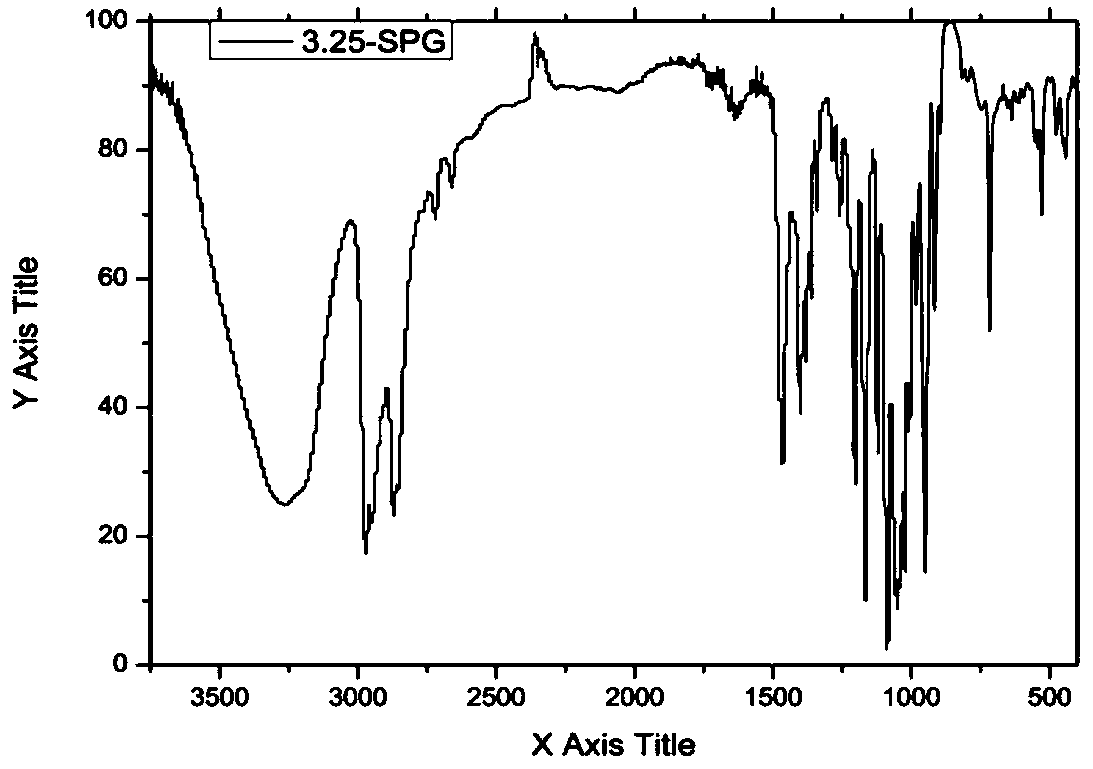
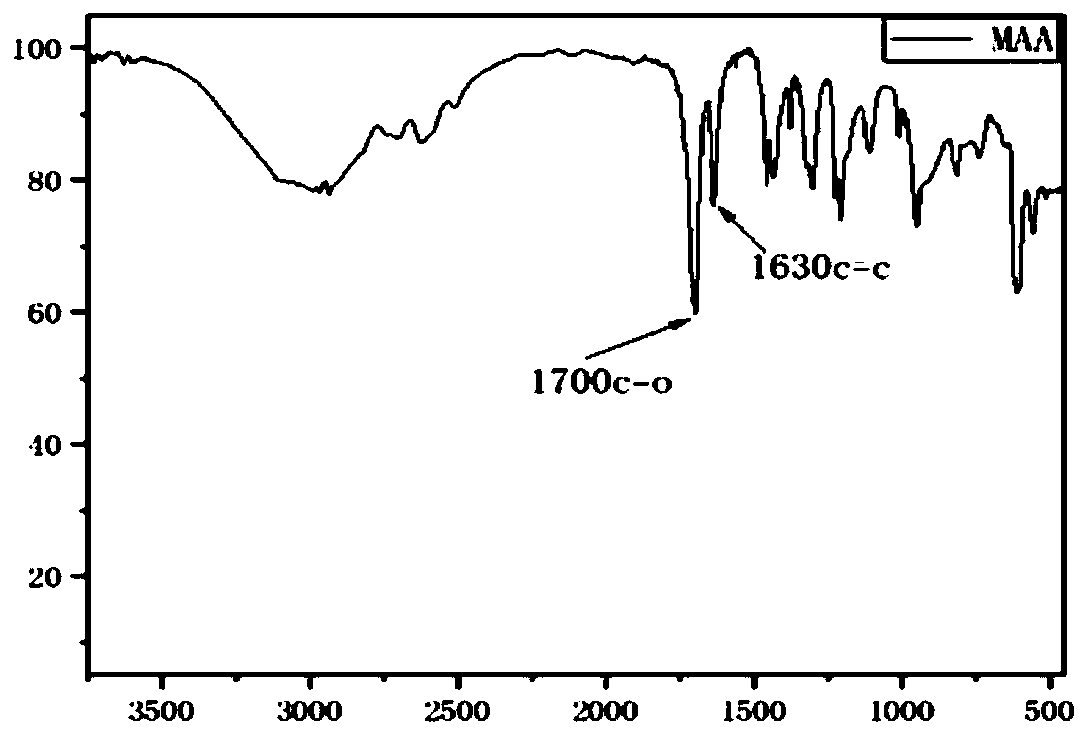
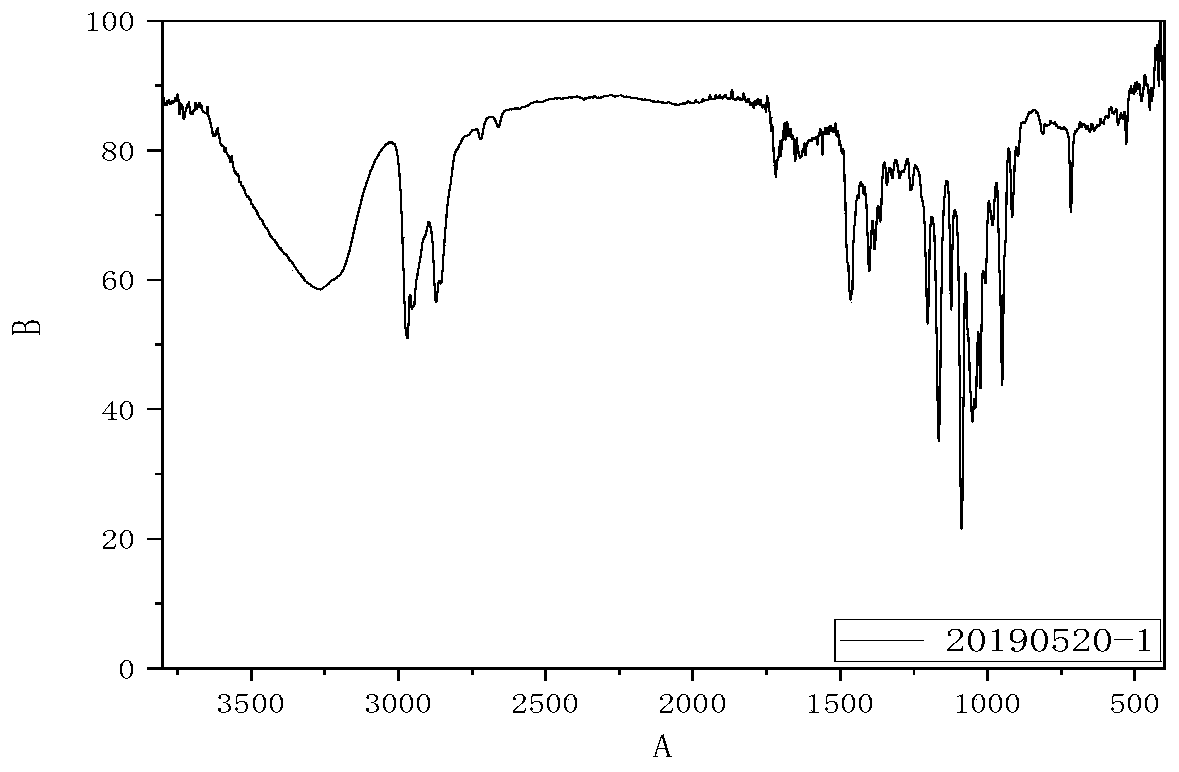
Spirodiol dienoic acid ester and preparation method thereof
A technology of spirodiol dienoate and spirodiol, which is applied in the field of diol ester compounds and their preparation, and in the field of spirodiol ester compounds and their preparation, can solve the problem of low product purity and yield, Problems such as secondary pollution and difficult recovery of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In the present embodiment, a kind of preparation method of spirodiol dienoate comprises the steps:
[0036] a. Pour a certain amount of reactant A spirodiol (SPG) into a 250ml three-necked flask, then add 20mL of mixed solvent, the volume ratio of toluene and tetrahydrofuran in the mixed solvent is 9:1, connect the three-necked flask to the condenser device, heat the three-necked flask to 65-75°C and stir for a certain period of time. After the spirodiol is completely dissolved and the solution becomes clear and transparent, add a small amount of anhydrous copper sulfate and p-hydroxyanisole, and then add the reactant methacrylic acid , finally add catalyst p-toluenesulfonic acid, make spirodiol and enoic acid as reactants, mix with mixed solvent to form reactant system; wherein the quality of anhydrous copper sulfate, p-hydroxyanisole and p-toluenesulfonic acid is 0.0083g respectively , 0.009g and 0.059g, the moles of spirodiol and methacrylic acid are respectively 0.0...
Embodiment 2
[0044] This embodiment is basically the same as Embodiment 1, especially in that:
[0045] In the present embodiment, a kind of preparation method of spirodiol dienoate comprises the steps:
[0046] a. Pour a certain amount of reactant A spiroglycol (SPG) into a 250ml three-necked flask, then add 20mL of mixed solvent, the volume ratio of toluene and tetrahydrofuran in the mixed solvent is 3:1, connect the three-necked flask to the condenser device, heat the three-necked flask to 65-75°C and stir for a certain period of time. After the spirodiol is completely dissolved and the solution becomes clear and transparent, add a small amount of anhydrous copper sulfate and p-hydroxyanisole, and then add the reactant methacrylic acid , finally add catalyst p-toluenesulfonic acid, make spirodiol and enoic acid as reactants, mix with mixed solvent to form reactant system; wherein the quality of anhydrous copper sulfate, p-hydroxyanisole and p-toluenesulfonic acid is 0.0085g respectively...
Embodiment 3
[0052] This embodiment is basically the same as the previous embodiment, and the special features are:
[0053] In the present embodiment, a kind of preparation method of spirodiol dienoate comprises the steps:
[0054] a. Pour a certain amount of reactant A spiroglycol (SPG) into a 250ml three-necked flask, then add 20mL of mixed solvent, the volume ratio of toluene and tetrahydrofuran in the mixed solvent is 3:1, connect the three-necked flask to the condenser device, heat the three-necked flask to 65-75°C and stir for a certain period of time. After the spirodiol is completely dissolved and the solution becomes clear and transparent, add p-hydroxyanisole as a polymerization inhibitor, then add the reactant methacrylic acid, and finally add Catalyst p-toluenesulfonic acid, make spirodiol and alkenoic acid as reactant, mix with mixed solvent to form reactant system; Wherein the quality of p-hydroxyanisole and p-toluenesulfonic acid are respectively 0.059g and 0.0176g, spirodi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com