Preparation method of cyclic phosphonate flame retardant
A cyclic phosphonate and flame retardant technology, which is applied in flame retardant fibers, chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, etc., which can solve the problems of catalyst pollution and long reaction time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
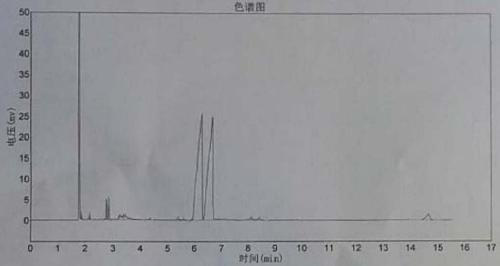
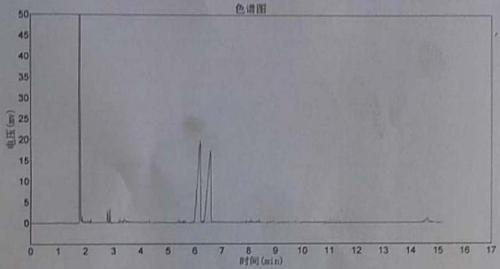
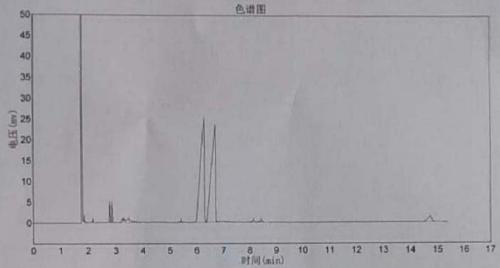
Image
Examples
Embodiment 1
[0037] Transesterification: Add 745g of trimethyl phosphite and 201g of trimethylolpropane to a 1000mL four-necked flask equipped with a magnetic stirrer, atmospheric distillation device (with packed column) and a thermometer, and heat to 80-120°C , Distillation and separation of by-product methanol while reacting, the column top temperature of the extracted methanol fraction is 63-65°C, and the reaction distillation is 4h to obtain the transesterification product;
[0038] Rearrangement: replace the packing column with a reflux condenser, so that the reaction distillation unit becomes a reflux reaction unit. Add 6g of catalyst methyl p-toluenesulfonate into the reactor, stir, heat to 170-180°C, keep warm for 2h to obtain the crude product;
[0039] Distillation under reduced pressure: transfer the crude product to a rotary evaporator, evaporate at a vacuum degree of 0.095-0.1MPa, and an oil bath temperature of 190-200°C, collect and obtain 390g of dimethyl methylphosphonate, ...
Embodiment 2
[0041] Transesterification: Add 558g of trimethyl phosphite and 201g of trimethylolpropane into a 1000mL four-neck flask equipped with a magnetic stirrer, atmospheric distillation device (with packed column) and a thermometer, and heat to 80-120°C , distill and separate the by-product methanol while reacting, the column top temperature of the extracted methanol fraction is 63-65°C, and the reaction distillation is 3h to obtain the transesterification product;
[0042] Rearrangement: replace the packing column with a reflux condenser, so that the reaction distillation unit becomes a reflux reaction unit. Add 5g of catalyst methyl benzenesulfonate into the reactor, stir, heat to 170-180°C, keep warm for 3 hours to obtain the crude product;
[0043] Distillation under reduced pressure: transfer the crude product to a rotary evaporator, evaporate at a vacuum degree of 0.095-0.10MPa, and an oil bath temperature of 180-190°C, collect and obtain 204g of dimethyl methylphosphonate, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Damage length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com